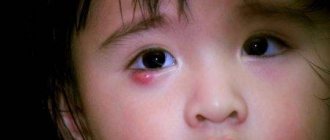
Hemangioma is a benign vascular tumor. It forms on the skin and internal organs. This disease may be congenital or appear within the first month after birth.
In some cases, for hemangioma, it is recommended to use Arutimol - eye drops for glaucoma. The effectiveness of treatment directly depends on the correct use of this drug and the severity of the disease. If you follow the instructions, you can get rid of hemangioma within 2 weeks.
Pharmacological authorities
Pharmacodynamics
.
Timolol is a non-selective β-blocker that does not have sympathomimetic or local anesthetic (membrane stabilizing) effects. Timolol inhibits β1 receptors, which are especially localized in heart cells, as well as β2 receptors.
Timolol reverses the stimulating effect of catecholamine on the heart. As a result, the conductivity of the atrioventricular block improves, the heart rate and cardiac output change. Blockade of β-adrenergic receptors in the bronchi and bronchioles leads to increased respiratory resistance through the presence of an antidote for parasympathetic activity.
Mіstseva action on the eyes.
Eye drops Arutimol® change both movement and normal internal eye pressure (HERE).
The exact mechanism of action of timolol, which manifests itself in altered intraocular pressure, is unknown until now. However, fluorophotometric and tonographic studies indicate that the effect of timolol may be due to changes in aqueous secretion. Several studies also showed redness in the watery veins.
In some patients, after treatment, there was a decrease in sensitivity to timolol, as well as to other drugs that reduce internal eye pressure.
A long-term study involving 164 patients followed for at least 3 years showed that after stabilization of the internal ocular pressure was achieved, there were no significant changes in the pressure.
In response to mitotic conditions, timolol changes the internal eye pressure without affecting the accommodation and size of the eye. The presence of miosis is especially important for patients with cataracts. When a patient switches from mitotic conditions to timolol, it is necessary to correct the refraction after the change in mitotic conditions.
Pharmacokinetics
.
The effect of the drug begins, as a rule, 20 weeks after local stagnation, reaching a maximum after approximately 1–2 years. The effect lasts up to 24 years.
The concentration of timolol in the first and second year after administration of 2 drops of timolol 0.5% becomes 150 ng/100 mg. Over 7 years, the concentration level changes to 10 ng/100 mg.
Radioactivity equivalent to 1–10 ng timolol per 100 mg tissue was recorded in the cornea, third region, iris/ciliary body.
Systemic absorption: studies have shown that timolol is chemically absorbed after intramuscular administration. Timolol was found in a cross-section of all healthy volunteers and patients (timolol maleate and its metabolites are excreted mainly by nitric acid).
Rhubarb in the blood: the concentration of timolol in the blood plasma after intramuscular stagnation of recommended clinical doses is often impossible to measure (less than 2 mg/ml), and neither after a single dose nor after a double dose.
The maximum concentration in blood plasma after instillation of 2 drops of the drug 2 times a day became 9.6 ng/ml 30–90 days after instillation.
Treatment of hemangioma
The drug Arutimol can be used to eliminate any forms and types of hemangiomas. But there are cases when such drug treatment does not always have an effect. This is affected by:
- patient's age,
- form of the disease
- using the product not according to the instructions.
This medicine should be used after consulting a doctor and undergoing diagnostics. These measures are necessary to determine the thickness of the tumor.
The solution is applied to the hemangioma using a spot method 3 times a day. The break between procedures is 8 hours.
Attention! To avoid getting the drug into the oral cavity, it should not be applied to the surface of the lips.
To monitor the effectiveness of Arutimol treatment, it is recommended to photograph the problem area on the skin every week. In addition, the patient must periodically undergo ultrasound diagnostics and a cardiogram. Through such procedures, it is possible to exclude heart problems at an early stage of their development.
During the period of treatment of hemangioma with this drug, the child should not receive preventive vaccinations for 3 months. The exception is staged vaccinations.
The therapeutic course lasts three months. Depending on the form and severity of the disease, this period can be extended to six months. If the treatment was carried out correctly, the neoplasm on the skin completely disappears or a spider vein remains.
Treatment recommendations:
- A solution of the drug (5%) should be applied to the problem area three times a day with an interval of 8 hours. Contact with skin should be at least 15-20 minutes.
- Every week it is necessary to record the dynamics of treatment in a photo. The first such registration is carried out before treatment.
- It is imperative to undergo an ultrasound and ECG of the heart for early diagnosis of diseases. In 15-20% of infants with hemangiomas, heart defects are detected.
- During treatment, do not carry out preventive vaccinations, with the exception of stage ones, for example, against hepatitis.
- A month after the start of therapy, it is necessary to undergo a medical examination.
The doctor evaluates the result after the procedures only after examination and local diagnostics.
Contraindicated
Arutimol®, ophthalmic drops, is contraindicated if the following illnesses are evident:
- bronchial hyperreactivity;
- bronchial asthma or a history of bronchial asthma;
- chronic obstructive illness of respiratory diseases;
- sinus bradycardia;
- weak sinus syndrome, sinoauricular block;
- atrioventricular (AV) blockade of another or third stage, which is not controlled by the pacemaker;
- cardiac failure is evident;
- high level of AV block;
- persistent heart failure;
- cardiogenic shock.
Arutimol®, ophthalmic drops, 0.25%/0.5% is also indicated in cases of hypersensitivity to any of the components of the drug, in severe allergic rhinitis or dystrophic disorders of the cornea.
The use of local β-blockers, such as timolol, and oral or intravenous administration of drugs of the calcium antagonist group to patients suffering from heart failure may result in a risk of injury. trioventricular conduction, left-sided heart failure and hypotension.
Treatment of patients with damaged cerebral hemorrhage should be carried out carefully. If, after treating eye drops with timolol, symptoms of reduced blood flow to the brain are avoided, alternative methods of treatment should be considered.
Treatment of patients with myasthenic weakness should be carried out carefully: when ophthalmic drops are dried with timolol, episodes of hyperemia of myasthenia, as well as myasthenic symptoms, are avoided. blurred eyes, drooping eyelids and profound weakness.
It is not recommended to use drops of timolol for the treatment of patients who are afraid of increased eye pain at night.
Interactions with other medicinal drugs and other types of interactions
No special studies of interactions with timolol have been carried out.
The immediate suppression of eye drops, which replaces adrenaline or pilocarpine, will enhance the effect of timolol to reduce internal eye pressure.
With one-hour administration of β-adrenergic receptor blockers, a bilateral enhancement of the effect is possible: on the eyes (reduction of internal ocular pressure) and on the cardiovascular system.
Nerve-muscle blockade caused by tubocurarine can be achieved by inhibiting the β-receptor (medicine Arutimol®, ophthalmic drops, 0.25%/0.5%). With one-hour administration of β-blockers and β2-sympathomimetics, the effect of β2-sympathomimetics may decrease and the appearance of important bronchospasms.
Immediate use of insulin or other antidiabetic agents, especially during stress or physical exercise (hypoglycemia), may cause or promote a lack of glucose in the blood, the symptoms of which may be masked .
Episodes of enhanced systemic β-blockade (eg, decreased heart rate, depression) have been reported during combined treatment with CYP2D6 inhibitors (eg, quinidine, fluoxetine, paroxetine) and timolol.
It was reported that the dilatation was due to the one-hour administration of ophthalmic β-blockers and adrenaline (epinephrine). There is a significant potential for additive effects that lead to hypotension and/or severe bradycardia when ophthalmic β-blockers are combined simultaneously with oral calcium channel blockers, β-adrenergic blockers ators, antiarrhythmic drugs (zocrema amiodarone), digitalis glycosides, parasympathomimetics, guanethidine. The nature of any cardiovascular adverse effects usually depends on the type of calcium channel blocker. Dihydropyridine-containing drugs, such as nefedipine, may lead to hypotension, while verapamil or diltiazem may be more likely to lead to impaired AV conduction or insufficiency of the left sac. when taken with a beta blocker.
Internal calcium channel blockers should be administered with caution to patients who inhibit beta-adrenergic blocking properties. Acute administration of beta-adrenergic blocking agents and digitalis with diltiazem or verapamil may have additive effects with prolonged periods of AV conduction.
Features of good stagnation
Tile for ophthalmological treatment.
During the hour of treatment with Arutimol®, ophthalmic drops, 0.25%/0.5%, it is necessary to regularly apply pressure to the internal eye and cornea.
Like other topical ophthalmic drugs, timolol is absorbed systemically. β-adrenergic component Arutimol®, ophthalmic drops, 0.25%/0.5% may cause the same side effects as side effects of the cardiovascular system, legenіv and other side effects such as side effects occur when systemic beta-adrenergic blockers are used. The frequency of systemic adverse reactions with local ophthalmic stasis is lower than with systemic stasis.
The heart is broken on the side.
It is necessary to critically evaluate the usefulness of therapy with β-blockers for patients with cardiovascular diseases (for example, ischemic heart disease, Prinzmetal's angina and heart failure) and hypotension and There is a possibility of therapy with other active substances. It is necessary to monitor patients with cardiovascular diseases for signs of deterioration in their condition, as well as the possible occurrence of adverse reactions.
Due to the negative influx for the hour of the pulse, β-blockers should be prescribed with caution to patients with stage 1 heart block.
Heart failure is to blame but must be properly monitored before starting therapy with Arutimol® drops. Patients with a significant history of heart disease should be monitored for signs of heart failure and their pulse rate monitored.
Damage on the side of the ships.
Care should be taken to treat patients with severe damage to peripheral blood flow (for example, a severe form of Raynaud's disease or Raynaud's syndrome).
Damage to the side of the organs, dihannia.
Respiratory reactions have been recorded, including fatal episodes through bronchospasm in patients with asthma after the administration of certain ophthalmic β-blockers. Arutimol® should be used with caution in patients with chronic obstructive illnesses of mild or moderate severity, and rarely, since the potential pain outweighs the potential risk.
Other β-blockers.
The effects of systemic beta-blockade may increase when timolol is prescribed to patients who are already discontinuing systemic beta-blockers. It is necessary to carefully monitor for similar reactions in such patients. It is not recommended to administer two local β-blockers at the same time (section “Interactions with other drugs and other types of interactions”).
Hypoglycemia/diabetes.
β-blockers should be used with caution in patients susceptible to spontaneous hypoglycemia, or in patients with mild diabetes, as β-blockers may mask the signs and symptoms of acute hypoglycemia mii.
Hyperthyroidism.
β-blockers may mask signs of hyperthyroidism.
Illness of the cornea
.
Ophthalmic β-blockers may cause dry eyes. Care should be taken when treating patients with corneal diseases.
Anaphylactic reactions.
When taking β-blockers, patients with a history of atopy or an important anaphylactic reaction to various allergens may be more sensitive until re-stagnation of such allergens and insensitive to high doses of adrenaline, such as rejoice in anaphylactic reactions.
Removal of the ship's shell.
It has been reported that the vascular membrane has been removed during stagnation therapy, which has been reported to suppress intraocular drainage (eg, timolol, acetazolamide) after trabeculotomy.
Anesthesia during the hour of surgical procedures.
Ophthalmic β-blockers can block the systemic action of β-agonists, such as adrenaline. It is necessary to inform the anesthesiologist that the patient is taking timolol.
Benzalkonium chloride
You may cause teasing of the eyes. Avoid contact with soft contact lenses. Benzalkonium chloride can cause a change in the color of soft contact lenses. Contact lenses should be removed before instillation of the medicinal product Arutimol®, ophthalmic drops, 0.25%/0.5% and re-attached no earlier than after 15 weeks.
Myazova's weakness.
It has been shown that beta-adrenergic receptor blockers cause muscle weakness, which is associated with the common symptoms of myasthenia (eg, diplopia, ptosis and general weakness).
There was also information that timolol causes muscle weakness in some patients with myasthenia gravis
or symptoms of myasthenia.
In patients with closed cuta glaucoma, the immediate method of treatment is to re-open the cuta. This is due to the sounding of the cells by miotics. Arutimol® hardly ever flows into the air.
When Arutimol® eye drops are cured to reduce the displaced internal eye pressure in case of glaucoma due to glaucoma, the following should be cured miotically, and not individually.
As with other antiglaucoma medications, some patients experienced decreased sensitivity to timolol after continued therapy.
Before general anesthesia, a step-by-step injection of β-adrenergic receptor blockers is administered, as they reduce the ability of the heart to respond to stimulation of sympathetic system receptors by β-adrenergic receptors.
If you want to further consolidate other personal features, you need to put 15 items between them.
During the hour of stabilization of the internal eye pressure, the decrease in the cob can become up to 50%, after which the effectiveness of the drug may change.
Patients should be warned not to allow the dispenser tip to come into contact with the eye or other structures.
Patients should also be warned that if they are not handled correctly, they may become infected with serious bacteria, which are known to cause infections. Serious damage to the eye and further waste of the eye may be the result of the subsequent destruction of erroneous affairs.
Patients should also be warned that in the event of any intercurrent condition of the eyes (for example, trauma, eye surgery or infection), they will immediately seek advice from a doctor further Wow, vikoristannya of an obvious high-dose container.
Bacterial keratitis has been reported as a result of stagnation of agaric containers of topical ophthalmic drugs. These containers were occasionally contaminated by patients, who in most cases suffered from concurrent congestion of the cornea or damage to the surface epithelium of the eye.
Suspension during pregnancy or breastfeeding.
Vaginism
There is not enough data on the use of timolol by pregnant women. Timolol maleate should not be used during pregnancy, as there are no clear indications for its use.
Epidemiological studies did not reveal malformative effects, but did demonstrate a risk of obstruction of intrauterine development when β-blockers were administered orally. In addition, signs and symptoms of β-blockade (eg, bradycardia, hypotension, suppressed breathing, and hypoglycemia) were observed in newborns when β-blockers were stopped completely. As soon as it is possible to freeze it until the curtains, then carry out close monitoring of the newlyweds during the first few days of their life.
Anniversary breastfeeding
β-blockers pass into breast milk and can cause serious adverse effects in cats who are breastfed. However, when therapeutic doses of timolol are administered in eye drops, it is unlikely that significant amounts of milk will be consumed in breast milk to cause clinical symptoms of β-blockade in animals. Recognizing the importance of measles during infancy for a child and measles during therapy for a woman, follow up with the use of the drug Arutimol®, ophthalmic drops, 0.25%/0.5%.
This is due to the fluidity of the reaction during treatment with vehicles or other mechanisms.
When properly frozen, the medicinal drug Arutimol®, ophthalmic drops, 0.25%/0.5% can be infused onto the surface, so it can be responsive when used in vehicles, robots with other mechanisms or robots. and in unsafe minds. Temporary blurred vision or other visual disorders, including refractive errors, diplopia, and ptosis, often result in mild and temporary blurred vision if there is blurred vision or increased windiness. This can be applied to the building using transport or various mechanisms. With one-hour exposure to alcohol, similar effects may become stronger.
Since the instillation results in blurred vision, the patient must wait until the vision becomes clear, first of all with a car or with machinery.
Method of congestion and dosage
For instillation into the eyes.
Instill eye drops at the lower conjunctival sac.
Apply 1 drop of Arutimol®, ophthalmic drops, 0.25% or Arutimol®, ophthalmic drops, 0.5% per dose onto the cob.
If the internal eye pressure is installed on each level during an hour of regular examinations, the dose can be changed to 1 drop of Arutimol®, ophthalmic drops, 0.25% or Arutimol®, ophthalmic drops, 0.5% 1 time per dose.
Like all antiglaucomatous drugs, Arutimol®, ophthalmic drops, 0.25% / 0.5% are intended for long-term stasis.
The reduction in pressure can become up to 50%, and then the effect may change (tachyphylaxis). In the period from the 3rd to the 12th month, the decrease in pressure stabilizes. Therefore, it is important to regularly check the pressure, especially in the first days after the stoppage of timolol eye drops. With oral administration of β-blockers, there is a decrease in internal ocular pressure, so it is important to note that local administration of timolol is still necessary. With systemic ingestion of β-blockers, the supplementary effect of local ingestion is, as a rule, significantly less.
When the nasolacrimal occlusion is stiffened or when the eyelid is flattened by 2 degrees, systemic absorption decreases. This can reduce systemic side effects and increase the local effect.
In patients with a heavily pigmented iris, decreased pressure may be caused by numbness or less pain.
Children.
The safety and effectiveness of drug administration in children has not been established
Premature babies are silent.
Apnea occurred very rarely in newborns, which may be explained by the physical immaturity of these patients. It is not recommended to use this drug for the treatment of newborns and premature infants in connection with the possible infusion of timolol into the central nervous system. It was noted that in some cases, administration of eye drops to timolol for the treatment of newborns and premature infants led to a significant increase in the level of timolol in blood plasma equal to that of adults.
Interaction with other drugs
If Arutimol was prescribed simultaneously with adrenaline-based eye drops, then pupil dilation develops. It is not recommended to use an antiglaucoma drug with other drugs based on beta-blockers, calcium channel blockers, because the risk of developing systemic adverse reactions increases.
Arutimol is able to potentiate the hypoglycemic effect of insulin and oral medications used to treat diabetes mellitus. Therefore, if simultaneous administration is necessary, the level of glucose in the bloodstream should be monitored. Eye drops can enhance the effect of muscle relaxants. Therefore, before surgery under general anesthesia, you should stop using Arutimol for 2 days.
Overdose
When the drug is frozen according to the instructions, the possibility of toxic side effects is virtually eliminated. Signs of overdose include decreased arterial pressure, congestive heart failure, cardiogenic shock, bradycardia and cardiac arrest. In addition, dyspnea, bronchospasms, scolial-intestinal disorders, confusion of information and judgment may develop.
In the event of an overdose, follow the next step:
1. Wash the tube if it gets into the middle. Studies have shown that timolol is not easily dialysed.
2. Symptomatic bradycardia: atropine sulfate, 0.25 to 2 mg intravenously, followed by stasis to induce vagal blockade. If bradycardia is preserved, then isoprenaline hydrochloride should be administered internally carefully. In persistent seizures, the cardiac pacemaker may be stuck.
3. Hypotension: following the stagnation of sympathomimetic vasopressor functions, such as dopamine, dobutamine or norepinephrine. In persistent seizures, the effects of glucagon stagnation were reported.
4. Bronchospasm: after congestion, add isoprenaline hydrochloride. Additional therapy with aminophylline may be considered.
5. Heart failure: it is important to initiate initial therapy with digitalis, diuretics and acids. In persistent episodes, stagnation of internal aminophylline occurs. After this, if necessary, you can freeze glucagon, which is known to be red.
6. Heart block (another or third stage): then use isoprenaline hydrochloride or a pacemaker.
In the meantime, come and adapt to the skin’s skin irritation.
Can the drug be used in the treatment of children?
A substance such as timolol is included in drugs for the treatment of ophthalmic diseases. The drugs Arutimol and Oftan Timolol are no exception.
In addition to ophthalmological diseases, this medicine can be used to treat skin diseases, for example, hemangiomas. Preference is given to such drugs due to their high efficiency, low risk of side symptoms in case of external use and minimal harm to the child’s health.
This drug cannot be used to treat hemangioma in newborns, weak and premature children.
Treatment of skin pathology is recommended at an early stage of its development. The speed of onset of the positive effect depends on this.
The medicine is applied to the problem area of the skin and left for 15-20 minutes. This procedure is repeated 4 times a day. The duration of the therapeutic course is determined by the doctor after assessing the first results. On average, this course lasts 10-16 weeks.
If after treatment the defective spot on the skin does not become lighter, then the use of the product can be extended until the hemangioma is completely lightened.
With the help of Arutimol, it will not be possible to completely eliminate the formation. However, it is possible to achieve a reduction in size and stop its growth.
Side effects
Like other ophthalmic drugs for local constipation, timolol maleate is absorbed into the systemic circulation. This may cause unwanted reactions similar to those seen with systemic β-blocking agents. The frequency of systemic adverse reactions after local ophthalmic stasis is lower than with systematic stasis. Reported adverse reactions include those observed with the ophthalmic β-blocker class.
Additional adverse reactions were avoided during administration of ophthalmic β-blockers and may potentially occur during administration of Arutimol®. Additional adverse effects have been reported in clinical trials with systemic timolol maleate, and these may be offset by the potential effects of ophthalmic timolol maleate. There are also excessive side effects that are expected in the class of ophthalmic beta-blockers, and may potentially occur when administered with Arutimol®.
On the side of the immune system
Systemic allergic reactions
, including angioedema, urticaria, localized and generalized rashes, itching, anaphylactic reactions, exposed swelling, redness.
Anaphylactic reactions.
When taking β-blockers, patients with a history of atopy or severe anaphylactic reactions to various allergens may be more sensitive until re-stagnation of such allergens and insensitive to high doses of adrenaline, such as rejoice in anaphylactic reactions.
On the side I exchange speeches and food
Hypoglycemia/diabetes, hyperglycemia. The ’-blocker is the Simultations of the GIPOGLIKEMI, Patzihntam, the cheeky to spontaneously gipoglikes, that to the Patziytam to the β-blocker of the Slid, to recognize the Ostrovarysteu.
Hyperthyroidism
β-blockers may mask signs of hyperthyroidism. Nonspecific thrombocytopenic purpura.
Mental disorders
Insomnia, depression, nightmares, loss of memory, hallucinations.
Systemic: decreased concentration, increased dreaming.
On the side of the nervous system
Syncope, impaired cerebral blood flow, cerebral ischemia, increased symptoms of myasthenia gravis
, confusion, paresthesia, headache, migraine.
Systemic: vertigo, local weakness.
Damage to the side of the organs
Impaired vision, including refractive changes (as a result of miotic therapy in some cases), diplopia, ptosis and choroidal vision after filtering surgery (chapter “Peculiarities for stosuvannya"). Signs and symptoms of eye irritation (for example, liver, prickling, itching, lacrimation, redness), blepharitis, keratitis, punctate keratitis, pain in the eyes, iritis, conjunctivitis, decreased vision acuity, photophobia, dryness eyes, vision from the eyes, itching eyes, thinning at the edges of the eyelids, burning of the anterior chamber of the eye, swelling of the eyelids, conjunctival hyperemia, uveitis, asthenopia, eczema of the eyelids, erythema of the eyelids, swelling of the eyelids, swelling of the conjunctiva, pigmentation I have corneas, blurred vision, decreased sensitivity of the cornea, erosion horns. Episodes of calcification of the cornea have been reported even rarely in connection with stagnation of eye drops to replace phosphates in some patients with a significantly damaged cornea.
Illness of the cornea. Ophthalmic β-blockers may cause dry eyes. Use caution in patients with corneal diseases.
On the side of my heart
Bradycardia, discomfort in the chest area, pain in the chest, rapid heart rate, swelling, swelling of the face, redness, arrhythmia, congestive heart failure, atrioventricular block, heart palpitation, heart failure activities.
Systemic: atrioventricular block (other or third stage), sinoatrial block, swelling of the legs, increased arterial insufficiency, decreased angina, vasodilation.
In patients with cardiovascular diseases (for example, ischemic heart disease, Prinzmetal's angina and heart failure) and arterial hypotension, it is important to carefully evaluate treatment with β-blockers and consider Bathing with preparations containing other potent substances. It is necessary to monitor patients with cardiovascular diseases for signs of deterioration in their condition, as well as the possible occurrence of adverse reactions. Through a negative influx for an hour of carrying out the pulse, β-blockers are prescribed with great care to patients with stage I heart block, myocardial infarction, and changes in arterial pressure.
On the side of the ship
Hypotonia, stroke, ear noise, claudication, Raynaud's phenomenon, cold endings. Care should be taken to treat patients with severe damage to peripheral blood flow (for example, a severe form of Raynaud's disease or Raynaud's syndrome).
On the side of the dicholic system
Asthma, bronchitis, chronic obstructive illness, stridor. Timolol should be used with caution in patients with chronic obstructive illnesses of mild or moderate severity, or less severely, since the potential cost outweighs the potential risk.
Systemic: wheezing.
Respiratory, thoracic and mediastinal disorders
Bronchospasm (important in patients with already obvious bronchospastic disease), shortness of breath, cough, nasal congestion, respiratory failure.
Shlunkovo-intestinal disorders
Loss of taste, boredom, dyspepsia, diarrhea, dry mouth, abdominal pain, vomiting.
On the side of the skin and under the skin fabrics
Lesions, psoriasis-like rashes or acute psoriasis, scalp rashes. Systemic: diarrhea, exfoliative dermatitis, systemic erythematosus.
On the side of the musculoskeletal system and tissue
Pain in the muscles, arthropathy, arthralgia, pain in the joints.
Damage to the side of the reproductive system and milk spots
State dysfunction, such as impotence, decreased libido, Peyronie's disease.
Systemic: complicated sechovypuskannya.
The destruction of the zagal character
Asthenia/increased fatigue, decreased physical vitality, increased sweating, exfoliative dermatitis, drowsiness, decreased secretion.
Systemic: pain at the end, decreased tolerance to physical rights.
Some patients with significantly damaged corneas rarely experienced episodes of corneal calcification through phosphate, which is located in eye spots.
After treatment, the effect of the drug may last for several days. If you treat with timolol, ophthalmic drops, apply after dry stagnation, this can be done for 2-4 days to reduce the internal pressure. When instilled in one eye, β-blockers can reduce intraocular pressure in the non-glare eye as well.
Contraindications for Arutimol
The medication is prohibited for use if there is hypersensitivity to the main component. A similar feature can develop with direct use of the medicine.
Contraindications:
- chronic forms of obstructive pathologies of the respiratory system, including bronchial asthma;
- the presence of a slow heartbeat - sinus bradycardia;
- development of heart failure;
- the occurrence of cardiogenic shock;
- severe form of atrophic rhinitis;
- 2nd or 3rd degree AV block;
- dystrophic changes in the cornea of the eye;
- age under 18 years;
- when carrying a child and during breastfeeding.
With great caution, the pharmaceutical product is prescribed to patients with chronic forms of heart failure, diabetes mellitus , and pulmonary failure.
This group also includes patients with hypoglycemia, suffering from thyrotoxicosis, myasthenic syndrome and Raynaud's syndrome, as well as those with pheochromocytoma. The drug should not be taken with the simultaneous prescription of other medications belonging to the group of adrenergic blockers. This can provoke a sharp drop in blood pressure and cause general weakness of the body.