In our NEW VISION Krasnodar clinic, Lucentis is administered . In ophthalmology, the administration of Lucentis is very effective in reducing visual acuity in certain diseases.
The clinical activity and effectiveness of this therapy has been proven in international trials of anti-VEGF drugs. When treated with Lucentis, the patients studied were able to stop the progression of the disease and even in most cases significantly improve visual acuity.
Composition, release form
Lucentis is a human monoclonal antibody fragment to endothelial growth factor A (VEGF-A) that is expressed by a recombinant strain of Escherichia coli.
1 bottle contains: active substance ranibizumab 10 mg/ml; 0.23 ml each.
Excipients: L-histidine hydrochloride, α,α-trehalose L-histidine, polysorbate 20, water for injection.
Available in the form of a solution for intraocular administration. Packaged in transparent, colorless glass bottles. The bottle is placed in a cardboard box along with a disposable syringe, a needle with a filter for extracting the solution, and an injection needle.
Reviews
A diagnosis of retinal dystrophy was made in the right and left eyes. I gave injections for free according to quotas. The next day after the procedure, my vision improved sharply, it became like in my youth, but after an hour and a half there was a significant decline. After completing the course, I began to see well in the distance, but I have to read with glasses. I had no allergy to the active substance (ranibizumab).
Sergey
I took Lucentis injections three times, the effect lasted for a month and a half and the swelling reappeared. After that I decided to try Ozurdex. After 10 days, the swelling decreased by half. The composition is valid from 3 to 6 months. I didn't have any contraindications.
Alina
I have already received 7 injections of Lucentis, and as a result the swelling has gone away. Time will tell how long this will last. The procedure feels no different from a regular injection ; it is only done in the operating room, with preliminary preparation of the eyes.
Alexander
pharmachologic effect
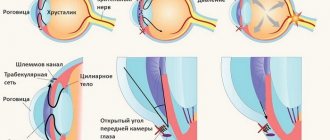
Lucentis suppresses the process of germination of newly formed blood vessels (neovascularization) into the retina of the eye in MDV. When using the drug, the thickness of the retina and its structure are normalized. Lucentis eliminates not only the consequences of MDV, but also the cause itself. The drug instantly penetrates all layers of the retina, reduces macular edema and the affected area, prevents new hemorrhages and pathological proliferation of blood vessels (angiogenesis).
Statistics regarding the use of the drug Lucentis are very optimistic: 90% of patients treated with the drug retained their vision, while 70% had better vision than before.
Indications for use of Lucentis
Since the main direction of action of the active components of the drug is to block the growth of pathogenic blood vessels in the retina of the eye, treatment with Lucentis is prescribed for the following indications:
- neovascular form of age-related macular degeneration
in patients of the older age group; - visual impairment associated with diabetic macular edema
; - in combination with laser coagulation can be used to treat vision loss
; - decreased visual acuity associated with choriodal neovascularization
caused by pathological myopia; - visual impairment caused by macular edema
caused by retinal vein thrombosis.
In other cases, the appropriateness of using the drug is determined by the attending physician based on the results of the examination and the characteristics of the disease.
Directions for use and doses
One vial of Lucentis is designed for only one intravitreal injection. The drug is administered into the vitreous body of the eye in the form of injections (intravitreal injection) in a dose of 0.05 ml (0.5 mg). The dose and mode of administration of the drug are selected by the attending ophthalmologist. At the first stage of treatment, the course consists of 3 injections, with an interval of 1 month; subsequently, to maintain and consolidate the effect, the interval between injections increases.
Chance of infection
A similar incident occurred in 2011 in the USA. 16 people in two states suffered severe eye infections after receiving Avastin, several of them going blind.
As experts explained in the American press, when dividing a standard “anti-cancer” dose of the drug into many small “ophthalmic” doses, there is a risk of infection.
Earlier that year, Avastin killed another patient, 77-year-old Lloyd Sylvis. During the injection, he was infected with streptococcus, which entered the brain through the eye. “He is now permanently blind and his brain is very damaged,” his son told the media.
In addition, in 2011, the US government revoked the license for the treatment of advanced breast cancer with Avastin. According to the Food and Drug Administration, there is no evidence that Avastin prolongs life or improves the quality of life of patients. At the same time, people taking Avastin risk a significant increase in blood pressure, extensive bleeding and hemorrhage, a heart attack, and heart failure.
Side effects
From the ophthalmology side: often - inflammation and detachment of the vitreous body, retinal and conjunctival hemorrhages, visual disturbances, eye pain, foreign body sensation, irritation and itching in the eyes, blepharitis, lacrimation, redness of the eyes, increased IOP.
Side effects from other systems are also observed, they manifest themselves in the form of cough, nausea, arthralgia, anxiety, anemia, headache, allergies, and rarely, stroke.
The drug is not for the treatment of eyes
Avastin (international nonproprietary name Bevacizumab) is commonly used to treat cancer. In Russia, it is not approved at all for the treatment of eye diseases - Vyacheslav Kurenkov, ophthalmologist, founder of the Ophthalmology Clinic of Dr. Kurenkov, editor-in-chief of the journal Ophthalmology, told Life about this.
“This drug is an antitumor drug,” he said. — Used for various cancers. Abroad, for example in Europe, it is also used in ophthalmology. In our country it is not used for the treatment of eye diseases, because in our country it is not registered as an ophthalmic drug. All medications undergo a certain procedure, and the Ministry of Health issues permission for use. And according to ophthalmology, Avastin simply did not undergo such a procedure.
Vyacheslav Kurenkov explained that analogues of Avastin, Lucentis and Elia, are used in ophthalmology in Russia. They are injected into the eye to treat diseases associated with changes in the retina.
“Perhaps Avastin was used at the Helmholtz Institute during clinical trials,” suggested Vyacheslav Kurenkov. - This is a scientific center - they have the right to use unregistered drugs.
According to him, complete loss of vision is not included in the list of side effects of this drug. So the reason, apparently, is not in the medicine itself.
“All drugs at the pharmaceutical enterprise are examined to ensure that they do not contain any infectious agents,” he said. — Special treatment is carried out, so it is almost impossible for infection to get inside. Perhaps there really was an infection if the sterility of the packaging was broken. For example, the seal may have been broken during transportation or storage.
At the same time, many experts, on the contrary, consider Avastin to be an effective remedy specifically for treating the eyes. Scientists from the Ufa Research Institute of Eye Diseases of the Academy of Sciences conducted a study on this topic back in 2010. Avestin treated 36 patients who had retinal vascular damage due to diabetes and retinal damage due to age-related loss of central vision.
As a result, visual acuity in all patients improved, as did functional indicators of the retina.
At the same time, the manufacturer of the drug, the Swiss company Roche Pharmaceuticals, opposes the use of Avastin in ophthalmology. It was previously reported that the company even conducted an internal investigation. She was approached by patients who began to have vision problems after doctors used Avastin to treat an ophthalmological disease.
In Russia, this drug is not sold in very large volumes. As Life was told by the DSM Group agency, which monitors the pharmaceutical sector, in total, since the beginning of 2021, 35 thousand packages of Avastin have been sold in Russia (for 1.6 million rubles). In 2015, 65 thousand packages were sold (3.4 million rubles).
Storage conditions and special instructions
Intravitreal injections should be performed by an experienced ophthalmologist. To prevent the occurrence of local infection, the patient should be under medical supervision for a week after administration of the drug.
In patients who have previously had a stroke, the risk of having a recurrent stroke after receiving Lucentis increases.
Lucentis weakens the effect of contraceptives, so women of childbearing age should take care of more reliable methods of contraception during treatment.
During the treatment period, it is advisable to refrain from driving and performing work that requires increased attention.
Victims' chances of recovery and compensation
Experts name three possible versions: a low-quality drug, a personnel error (the injection was given under unsterile conditions) or an infection. Ophthalmologist Dmitry Davydov assessed the chances of the victims:
“The worst-case scenario is complete loss of vision,” said Davydov. - Again, it depends on the reason. For a day, for a month, for six months - it’s hard to say yet. Even if it is an infection, then you need to understand what kind of infection it is - staphylococci, streptococci, Pseudomonas aeruginosa, gram-negative flora, how long it was there, in what strain of dilution, how the body reacted to it, whether measures were taken immediately. We do not yet know how long this situation lasts, or to what extent visual functions are reduced.
According to the chairman of the bar association “Your Legal Attorney” Konstantin Trapaidze, the victims could each receive up to 2 million rubles.
“If it is established that people have lost their visual function completely, then this will be considered serious harm to health,” he said. - It will be necessary to find out who is to blame - doctors, manufacturers or suppliers. Was there negligence here, or did the perpetrators know that the medicine, for example, was of poor quality? Victims can count on compensation for moral and material damage. Payments can reach a maximum of 1-2 million rubles. And this is not bad: 10 years ago, payments for causing harm to health were 30-40 thousand rubles. Now the amounts have become larger. I even heard of cases where patients received three and six million rubles.
By the way, the research institute has already promised to restore their sight to all victims.
How is an intravitreal injection performed?
On the eve of treatment, the patient must undergo computer vision diagnostics. After studying the results of the examination, the doctor draws up an individual treatment regimen with Lucentis.
Only highly qualified ophthalmologists of the clinic who have extensive experience in administering the drug into the vitreous body of the eye are allowed to carry out treatment with Lucentis. The procedure is performed on an outpatient basis; the patient does not need to be in the hospital. Usually, after the main course of treatment, a stabilization phase begins, however, to consolidate the results obtained, the patient needs to be seen by an ophthalmologist 2-3 times a year.
With the help of this remedy you can preserve, stabilize and improve vision in the wet form of macular degeneration.
Contraindications
The use of Lucentis is not recommended in the following cases:
- the patient has hypersensitivity to the active components of the drug;
- any infectious eye diseases have been previously diagnosed;
- The patient was diagnosed with intraocular inflammation.
It is not recommended to use this solution for the treatment of vision loss in children under 18 years of age, as well as in women during pregnancy and lactation.
Lucentis therapy should be prescribed with caution to patients with a previously documented allergic reaction and if there is a risk of a stroke. In these cases, medical supervision of the patient's condition during and after the procedure should be carried out.
Lucentis analogs
An analogue of Lucentis is the drugs Avastin (bevacizumab), partly: Ozurdex (dexamethasone for intraocular administration) and Vizudin (photosensitizer, used for neovascularization).