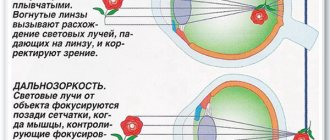
Eye
Lens
(lat. lens) - a transparent body located inside the eyeball opposite the pupil; is a biological lens, the lens is an important part of the light-refracting apparatus of the eye.
The lens is a transparent biconvex round elastic formation, circularly fixed to the ciliary body. The posterior surface of the lens is adjacent to the vitreous body, in front of it are the iris and the anterior and posterior chambers.
Dimensions and optical properties
The maximum thickness of the lens of an adult is approximately 3.6-5 mm (depending on the tension of accommodation), its diameter is about 9-10 mm. The radius of curvature of the anterior surface of the lens at rest of accommodation is 10 mm, and the posterior surface is 6 mm; at maximum accommodation stress, the anterior and posterior radii are equal, decreasing to 5.33 mm.
The refractive index of the lens is heterogeneous in thickness and averages 1.386 or 1.406 (core), also depending on the state of accommodation.
At rest of accommodation, the refractive power of the lens averages 19.11 diopters, at maximum accommodation voltage - 33.06 diopters.
In newborns, the lens is almost spherical, has a soft consistency and a refractive power of up to 35.0 diopters. Its further growth occurs mainly due to an increase in diameter.
Symptoms of lens diseases
With age, the structure of the lens changes: it becomes denser and reacts less well to the tension of the ligamentous apparatus. Because of this, Patients over 40 years of age often have complaints of decreased near vision, i.e., presbyopia develops.
Age-related changes and metabolic disorders lead to loss of transparency of the lens - cataracts form. The most common symptom of this disease is cloudiness in the eye: the image becomes yellowish and dim. There is a feeling that everything around is visible through cellophane film. Halos may appear when looking at light sources.
Age-related lens opacities progress rather slowly, up to several decades. Sometimes the cause of cataracts is not age, but long-term eye inflammation or glaucoma (increased intraocular pressure). Injuries to the eye can also cause clouding of the lens.
Diseases do not always make themselves known with clear symptoms, so we advise you to consult an experienced ophthalmologist for any changes, even the most minor ones.
Histological structure
a capsule in the lens
(bag),
capsular epithelium
and
the main substance of the lens
.
Capsule
On the outside, the lens is covered with a thin elastic structureless capsule, which is a homogeneous transparent shell that strongly refracts light and protects the lens from the effects of various pathological factors. The capsule is attached to the ciliary body using the ciliary girdle.
The thickness of the lens capsule is not the same over its entire surface: the front part of the capsule is thicker than the back (0.008-0.02 and 0.002-0.004 mm, respectively), this is due to the fact that on the front surface under the capsule there is a single layer of epithelial cells.
The capsule reaches its greatest thickness in two of its belts concentric to the equator - the anterior (located 1 mm inward from the place of attachment of the anterior fibers of the ciliary girdle) and the posterior (inward from the place of posterior attachment of the ciliary girdle). The thinnest capsule is in the region of the posterior pole of the lens.
Epithelium
The lens epithelium is characterized as single-layer squamous non-keratinizing; its main functions are trophic, cambial and barrier.
The epithelial cells corresponding to the central zone of the capsule (opposite the pupil) are flattened and tightly adjacent to each other. Almost no cell division occurs here.
As we move from the center to the periphery, there is a decrease in the size of epithelial cells, an increase in their mitotic activity, as well as a relative increase in the height of the cells so that in the equator region the lens epithelium practically turns into prismatic, forming the growth zone of the lens. Here the formation of so-called lens fibers occurs.
Lens substance
The bulk of the lens is formed by fibers, which are elongated epithelial cells. Each fiber is a transparent hexagonal prism. The substance of the lens, formed by the protein crystallin, is completely transparent and, like other components of the light-refracting apparatus, is devoid of blood vessels and nerves. The central, denser part of the lens has lost its nucleus, shortened, and when superimposed on another fiber it has become called the nucleus
, while the peripheral part forms a less dense
cortex
.
During intrauterine development, the lens receives nutrition from the vitreous artery. In adulthood, the nutrition of the lens is entirely dependent on the vitreous body and aqueous humor.
Diagnosis and treatment of eye lens diseases
To assess the condition and functioning of the lens, the method of biomicroscopy (non-contact examination using a slit lamp) is used. This is how an ophthalmologist determines the size of the lens, the degree of its transparency, and identifies the presence and location of opacities.
To treat cataracts, doctors at Dr. Belikova’s Eye Clinic use the most modern, safe and fastest method to date - ultrasound phacoemulsification. By replacing your clouded lens with an artificial IOL (intraocular lens), you can restore your vision to 100% and get rid of glasses forever!
For specialists
BIOMECHANICS OF ACCOMMODATION
Chapter from the book “Biomechanics of the Eye”
(E.N. Iomdina, S.M. Bauer, K.E. Kotlyar)
The results of Russian and foreign studies devoted to the study of the biomechanical structures of the eye are presented. The possibilities of using research results both to understand the pathogenesis of various eye diseases and in clinical practice are shown.
FULL TEXT OF THE BOOK AT https://sabar.eye-portal.ru
Biomechanics of the eye is not only one of the scientific areas of ophthalmology that allows us to better understand the functioning of the eye in normal and pathological conditions, but also an effective tool for the development of new diagnostic tools, optical and surgical correction of visual disorders. This chapter presents the main clinical applications of biomechanical studies of various structures and systems of the organ of vision.
Biomechanics of accommodation
Accommodation - the basis of normal visual perception - is a biomechanical system (with feedback) of the optical adjustment of the eye for clear vision of objects located at various distances, which includes, first of all, the lens, its ligamentous apparatus (zonular fibers), ciliary muscle and choroid. Other structures of the eyeball are also involved to varying degrees in the biomechanism of accommodation, namely the iris (pupillary sphincter), extraocular muscles, vitreous body, cornea, eyelids (accommodation guide).
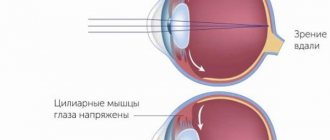
Today, the fundamental theory describing the mechanism of accommodation is the hypothesis of Hermann von Helmholtz, according to which, in order to obtain a clear image of objects located at a close distance, the following changes occur in the human eye: the ciliary muscle contracts, the pupil narrows, the depth of the anterior chamber decreases, the lens moves slightly anteriorly and downward, the tension of the ligaments of Zinn weakens, the radius of curvature of the anterior and posterior (to a lesser extent) surfaces of the lens decreases, which leads to an increase in its refractive power and an increase in the dynamic refraction of the eye (Helmholtz Hv, 1855).
Since the publication of Helmholtz's point of view on the mechanism of accommodation, attempts have not stopped to challenge, modify or supplement his theory, since not all clinical situations can be described from the perspective of this theory.
Modern theories of accommodation can be divided into intracapsular and extracapsular. The first group of theories is united by a common biomechanical principle: when the ligamentous apparatus (zonular fibers) is weakened, the elastic forces of the lens capsule lead to its rounding, i.e. to increase its refractive power (links). Proponents of the second group of theories believe that a change in the shape of the lens for clear vision of close objects is caused by the vitreous body moving forward, the hydraulics of the intraocular fluid of the posterior chamber, the tense equatorial ligaments of the lens, or a combination of these factors.
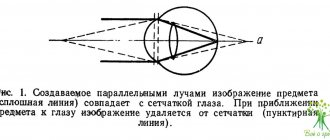
A serious argument in favor of supporters of the intracapsular accommodative mechanism was the results of carefully performed experimental work by a well-known specialist in the field of ocular accommodation A. Glasser (1998, 1999). He conducted an experiment on monkeys using ultrasonic biomicroscopy (UBM) to determine exactly how the equatorial diameter of the lens changes at the height of the accommodation stress, i.e. in the area of the nearest point of clear vision: increases or decreases (Fig. 1). If it increases or does not change, then supporters of deformation of the equatorial zone of the lens by any of the described force influences - the vitreous body, the ligamentous apparatus, or intraocular fluid - are right. If the diameter of the lens decreases, then supporters of the mechanism of changing the shape of the lens due to the elastic forces (elasticity) of its capsule are right, i.e. intralens structure.
The experiment showed a significant decrease in the equatorial diameter of the lens at the height of accommodation, thereby confirming the intracapsular hypothesis of Helmholtz and his supporters, who attach paramount importance in the mechanism of accommodation to the elastic forces of the lens structures.
At the same time, in addition to the dominant concept of “lens” accommodation, there are many opinions about the participation of other eye structures in this process.
In order to more fully understand the mechanism of accommodation, identify all its participants, develop on this basis effective means of clinical diagnosis of accommodation disorders and determine ways of adequate correction, three main possibilities are currently used: 1) computer modeling, 2) clinical observations of eye structures using modern visualization tools and 3) determination of certain clinical indicators of the accommodative ability of the eye.
Indeed, modern technical capabilities make it possible to create very productive virtual mechanical and mathematical (numerical and analytical) models, which become an effective tool for testing scientific hypotheses and resolving controversial issues, including accommodation.
In particular, the use of a biomechanical model of the eye (Iomdina E.N.) to study the mechanism of accommodation and its age-related changes showed that with age, with an increase in the rigidity of the lens structures and a change in the ratio of the rigidity of the nucleus and cortex of the lens (with the rigidity of the nucleus higher than the rigidity of the cortex), the lens loses its normal shape and deformability, as a result of which not only an age-related decrease in the volume of accommodation occurs, but also a change in the accommodative mechanism itself: the refractive power of the eye when accommodating near may be less than when accommodating at a distance, which does not correspond to Helmholtz’s theory of accommodation. In clinical practice, these age-related features must be taken into account when selecting optical correction, in particular presbyopic, planning cataract extraction and determining the parameters of an intraocular lens (IOL). Once the contents of the lens, including the hard nucleus, are removed, the accommodative ability of the eye may increase. This conclusion, obtained on the basis of biomechanical modeling, is confirmed, in particular, by the recently obtained results of a clinical study by Ovechkin I.G. et al. (Ovechkin I.G., Belikova E.I., Shalygina E.L., Antonyuk S.V., Ovechkin N.I. “Accommodative ability of the eye in patients after phacoemulsification of cataracts with implantation of monofocal, multifocal and accommodating intraocular lenses.” ROZH 2014), which showed the appearance of true accommodation (objective accommodative response) in patients with presbyopia after phacoemulsification of cataracts and implantation of monofocal, and especially accommodating IOLs. This conclusion is confirmed by the dynamics of the basic parameters of the accommodative response in operated patients and patients in a comparable control group without pathology of the organ of vision. The data obtained indicate that accommodating IOLs and (to a much lesser extent) monofocal IOLs, after removal of the lens contents and rigid nucleus, respond proportionally to ciliary muscle contraction with a displacement along the optical axis of the eye.
Calculations also show that with maximum accommodation stress (i.e., when focusing on the closest and clearly visible object) and with a sufficiently rigid lens, sagging of some portions of the zonular fibers can be observed, which is consistent with the results of UBM and may be a risk factor for the development of subluxation of the lens.
As stated above, great opportunities for clinical observation of the biomechanism of accommodation are provided by modern technologies for visualizing ocular structures, such as UBM, optical coherence tomography (OCT), magnetic resonance imaging (MRI) and Scheimpflug imaging.”
The photographs obtained by the “Scheimpflug imaging” method clearly show a change in the radius of curvature of the lens during accommodation (Fig. 2 a, b), as well as an increase in the curvature of its anterior surface in the central zone during distance accommodation, caused by the presence of a hard lens core (Fig. 2 V).
V.V. Strakhov, using a UBM (“Humphrey UBM-840” with a sensor oscillation frequency of 50 MHz), were able to identify significant age-related topographic-anatomical changes in the orbicular part of the posterior chamber of the eye, the tone and direction of the zonules of zonules, caused by an involutional increase in the size of the lens, which indicates that In the opinion of the authors, the importance of reducing the working distance of accommodation in the development of presbyopia (Fig. 3.). The author concludes that in the biomechanism of presbyopia, along with the well-known lens (Helmholtz theory) and muscular (Donders theory) concepts, a new one appears - ligamentous. It should be noted, however, that the lack of a three-dimensional picture of the anterior segment of the eye, which cannot be obtained using two-dimensional UBM scanning, does not yet allow us to put an end to the study of the real course of the zonular fibers and the volume of the anterior and posterior chambers of the eye during accommodation and requires further research on this issue.
It must be emphasized that the issue concerning the biomechanical role of the ligamentous apparatus of the lens in the operation of the accommodation mechanism is very important.
As is known, the zonular fibers of the ligament of cinnamon are divided into three main portions: the anterior portion of the fibers (APF), the posterior portion of the fibers (PPF) and cilioequatorial fibers (Fig. 4.). The first two portions of fibers originate from the dentate line. The detailed question of the posterior attachment of these fibers is discussed by modern researchers. The PPV is adjacent to the ciliary body, connecting to it with the help of short thin fibers up to the ciliary processes and, passing in groups between them, is attached to the anterior capsule of the lens 1.5-2.0 mm from its equator in separate fixed places around the circumference, unevenly , but by segments.
Segmental circumferential attachment of the “bundles” of the PPV of the ciliary girdle to the lens capsule can, in principle, allow the ciliary muscle to differently stretch the segments of the anterior surface of the lens capsule, partially compensating for the natural or acquired astigmatism of the optical system of the eye. It is possible that in this case the COM segments can contract with different intensities. This hypothesis (about uneven accommodation), formulated by Svetlova O.V. et al., awaiting experimental confirmation. The RPV is thicker and more powerful than other portions of zonular fibers. The ZPV is adjacent to the anterior limiting membrane of the CT, as if duplicating it with its network and connecting with it by a system of intertwined short thin fibers to the point of attachment to the posterior capsule of the lens, 1.0–1.5 mm from its equator. Here the ZPV is woven into the hyaloid-capsular ligament of Wieger. The fibers of the ZPV are thinner but more numerous than those of the PPV. The third portion of fibers, CEV, goes from the lateral surface of the bases of the ciliary processes to the equatorial capsule of the lens, where they are arched and evenly located along the equator of the lens along the line of their attachment. The CEV is weaker than the other zonular fibers and is least developed. In adulthood they often die off.
The participation of the ligamentous apparatus in the decrease in accommodative ability with age is confirmed by the data of R. Michael et al. (2012), who established a decrease in the elastic modulus of zonular fibers in people of older age groups.
The role of zonular fibers in the normal accommodative process and the development of its disorders is discussed in the study of D. Goldberg (2011), who formulated the theory of the coordinated synchronous (reciprocal) action of zonular fibers. The essence of this theory is that when the ciliary muscle contracts, the anterior portion of the fibers is weakened, and the posterior portion is stretched and exerts a force on the posterior capsule of the lens, changing its thickness and the shape of the posterior surface. During relaxation of the ciliary muscle, the posterior fibers are weakened, and the lens moves posteriorly due to increasing tension in the anterior ligaments (Fig. 5).
In addition to the lens and its ligamentous apparatus (zonular fibers), the ciliary muscle, of course, plays the most important role in the process of accommodation.
The ciliary muscle, which belongs to the category of smooth muscles, is conventionally divided into three portions (muscles of Brücke, Ivanov and Müller), in which the muscle fibers are oriented in different directions (Fig. 6).
The meridional Brücke muscle, the most powerful and longest (on average 7 mm), is attached to the sclera in the area of the corneo-scleral trabecula and scleral spur, freely extends to the dentate line, where it is woven into the choroid, reaching the equator of the eye in separate fibers. The generally accepted opinion is about the parasympathetic innervation of this muscle (“Accommodation. A guide for doctors, edited by L.A. Katargina, Moscow, 2012).
A portion of the internal fibers (Müller's circular muscle) does not have an attachment, like the iris sphincter, and is located in the form of a ring at the very apex of the crown of the ciliary body. When it contracts, the apex of the crown “sharpens” and the processes of the ciliary body approach the equator of the lens. It is generally accepted that the innervation of the circular muscle, like the meridional muscle, is parasympathetic.
Between these two portions are radial muscle bundles (Ivanov's radius muscle), which often form an interwoven lattice (Standring S., Borley N., Collins P., et al. "Gray's Anatomy: The Anatomical Basis of Clinical Practice." Chapter 40. The eye (Elsevier, 2009). This portion constitutes the main muscle mass of the crown of the ciliary body and, having an attachment to the uveal part of the trabecular meshwork in the basal zone of the iris, freely ends in the form of a radially diverging corolla on the back side of the crown facing the vitreous body.
It is obvious that during their contraction, the radial muscle fibers, being pulled to the place of attachment, will change the configuration of the crown and shift it towards the root of the iris. Despite the confusion of the issue of innervation of the radial muscle, most authors consider it sympathetic. L.A. Deev et al. (1996) discovered not only the presence of sympathetic synapses in the ciliary body, but even the accumulation of adrenergic nerve terminals in the area of the radial portion of the ciliary muscle.
The biomechanism that provides the entire volume of accommodation from the further to the nearest point of clear vision and, conversely, from the nearest point of clear vision to the further is under the influence of the sympathetic and parasympathetic parts of the autonomic nervous system and is realized through the active and passive muscle components due to the contraction of the radial Ivanov muscle (sympathetic innervation) and relaxation of the meridional Brücke muscle (parasympathetic innervation) and the circular Müller muscle (parasympathetic innervation).
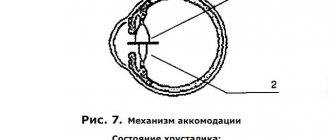
The question of the type of innervation of various portions of the ciliary muscle is important for the correct biomechanical representation of its work during the process of accommodation, for understanding the contribution of each portion to the accommodative mechanism and for developing means of targeted regulation of their functional activity (Fig. 7).
In this regard, the use of drug models of various tonic conditions of the ciliary muscle is fruitful. Thus, V.V. Strakhov et al., using instillations of drugs (anticholinergics, adrenergic agonists) that activate parasympathetic or sympathetic receptors and thereby affect various portions of the ciliary muscle, using UBM recorded changes in the ciliary body and the ligamentous apparatus of the lens, confirming the presence of active the mechanism of accommodation of the eye for both near and distance.
Undoubtedly, UBM and other imaging technologies (UBM, MRI, etc.) make it possible to record changes in the shape and geometric parameters of the muscle, its displacement during the process of accommodation, age-related and other features. However, it is also advisable to evaluate the functional characteristics of the ciliary muscle, its performance, and reactions to drugs or other effects using technical means of recording the accommodative response.
Research shows that the tone of the ciliary muscle is constantly changing. These fluctuations are called accommodative microfluctuations (AMF). AMFs have a specific frequency and consist of low- and high-frequency components. The low-frequency component (frequency less than 0.6 Hz) is background and has no clinical significance, and the high-frequency component (frequency between 1.0 and 2.3 Hz) reflects fluctuations in ciliary muscle fibers, and its measurement makes it possible to assess muscle contractility.
To study the AMF, a Righton Speedy-K ver.MF-1 accomodograph is used, which performs a frequency analysis of the AMF using the Fourier transform method. Computer accommodation allows a detailed assessment of the performance of the ciliary muscle, diagnosis of the functional state of accommodation, its dynamic changes, including against the background of therapeutic measures.
The study of accommodation using binocular “open field” autorefkeratometers, for example, Grand Seiko WR-5100K, WR-5500, is also automatic and objective. A distinctive feature of these devices is that, thanks to the field open to the subject’s gaze, the object of fixation is presented in real (rather than virtual) space, it is possible to move it from 20 cm to infinity (5-6 m), as well as attach various lenses to the subject’s eyes diopter, that is, measuring the accommodative response under defocusing conditions. Using this technique, it is possible to determine, in particular, the tone of accommodation, which is one of the important indicators of dynamic refraction. In the dark, i.e. in the absence of a stimulus for accommodation, some tone of the ciliary muscle is preserved, due to which the optical alignment of the eye corresponds to a point occupying an intermediate position between the nearest and further points of clear vision. The position of this point, also called the resting point of accommodation and determined by the difference in the values of the static (i.e., in conditions of cycloplegia) refraction of the eye and the dark focus of accommodation (i.e., refraction in the absence of an accommodative stimulus), can be influenced by the state of the autonomic nervous system ( balance of sympathetic and parasympathetic innervation) and a number of other factors. Determining this indicator is of great importance both for identifying the causes of accommodative disorders and eye diseases associated with these disorders, primarily myopia, and for determining adequate means of their correction.
The use of a set of methodological techniques, including visualization tools (UBM), registration of accommodation indicators using accommodography and an “open field” binocular autorefkeratometer, made it possible to study in detail the effect of a course of instillations of an adrenergic agonist - 2.5% irifrin solution (Sentiss, India) on the state of normal accommodation and with myopia. Irifrin, containing 25 mg of phenylephrine hydrochloride and 0.1 mg of benzalkonium chloride in 1 ml, has pronounced alpha-adrenergic activity and, when applied topically in ophthalmology, constricts blood vessels and dilates the pupil without causing cycloplegia. Scientific and practical interest in the drug is due to the fact that it is practically the only currently existing means of stimulating accommodation in the distance by influencing the sympathetic nervous system.
It has been shown that a course of 2.5% irifrin changes the tone of the ciliary muscle and the balance of autonomic innervation towards a decrease in the tone of the parasympathetic nervous system, causes a shift in dynamic refraction towards weakening, helps to increase the reserves of relative accommodation and the objective accommodative response, which can be explained by the effect of negative accommodation (accommodation for distance) and improving the performance of the ciliary muscle (Tarutta E.P., Iomdina E.N., Tarasova N.A., Filinova O.B. “The influence of 2.5% irifrin on accommodation indicators and refractive dynamics in patients with progressive myopia." Rozh, 2010, No. 2, pp. 30-33). When combining an effect on the sympathetic nervous system (drug stimulation with irifrin of accommodation for distance) with a simultaneous effect on the parasympathetic nervous system (functional training of accommodation upon presentation of a stimulus for near) and physiotherapeutic treatment that improves the hemodynamics of the eye, the accommodative ability impaired with myopia improves most significantly (Volkova E.M., Strakhov V.V. “Use of Irifrin as a stimulator of accommodation for distance.” Clinical Ophthalmology, 2005, No. 2, pp. 86-88).
The results obtained are confirmed by accommodation data using the Speedy-K ver. MF-1 (Egorova A.V., Mykolnikova E.S. “The drug Irifrin 2.5% in the treatment of computer visual syndrome.” Clinical ophthalmology, 2009, No. 1, 30-32).
Currently, irifrin is produced in the form of eye drops without a preservative (benzalkonium chloride), which significantly expands the possibilities of its safe and effective use for the correction of accommodative disorders and the prevention of myopia progression.
In addition to the main (active) participants in the accommodation mechanism - the lens and its musculo-ligamentous apparatus - the choroid also takes part.
Macroscopically, the border of the choroid and the ciliary body is the dentate line, ora serrata, which represents the border of the optical part of the retina. The choroid is a thin, soft, elastic brown membrane that is in a state of moderate tension. The thickness of the choroid in the region of the posterior pole of the eye is 0.22 mm, it gradually thins towards the periphery to 0.10 - 0.15 mm. Along its length, the choroid is adjacent to the sclera, and between them there is a narrow capillary gap - the suprachoroidal space. The posterior ends of the more deeply located meridional fibers of the CM pass into the elastic fibrils of the choroid. When the meridional fibers of the CM contract, this system of elastic fibers is stretched. The more superficially located fibers of the CM with their posterior ends are part of the epichoroid, a system of thin connective tissue plates located under the sclera. Through them, these muscle fibers are fixed directly to the inner surface of the sclera. Further posteriorly, with the help of similar, but shorter plates, the choroid itself is fixed to the sclera.
The participation of the choroid in the mechanism of accommodation is presented in the work (Svetlova O., Makarov F., Kotlyar K., Zaseeva M., Koshits I. Morphological and functional features of the design of the ciliary band of the lens as a key link in the mechanism of accommodation of the human eye. Morphology; 2003:7 -16.) (Fig. 8). The authors offer their description of accommodation at distance (disaccommodation) and near, assigning the choroid, according to the apt definition of A.I. Gorban, the role of a kind of biological spring. When looking into the distance, the meridional and circular fibers of the ciliary muscle relax, its radial fibers contract, “pulling apart” the circular fibers like an iris dilator. The elastic choroid returns (compresses) to its original (unstretched) state, pulling the site of posterior attachment of the fibers of the zonular ligament posteriorly. The anterior portion of the fibers of the ligament of Zinn is stretched and presses the lens to the vitreous body. In this case, the lens itself is pressed into the anterior limiting membrane (ALM) of the vitreous body, but the taut (and at this phase stretched over the surface of the ALM) posterior portion of the fibers of the ligament of Zinn prevents this depression. The lens is thus flattened by the force of the anterior portion of the fibers and is pressed against the elastically stressed surface of the posterior portion of the fibers and the PPM. This state is stable and well dampens possible fluctuations of the lens.
When viewed up close, the meridional and circular fibers of the ciliary muscle contract and its radial fibers relax. The ciliary body moves medially anteriorly, stretching the elastic choroid and thus pulling the place of posterior attachment of the fibers of the zonular ligament forward. The tension of the anterior and posterior portions of the fibers of the ligament of zinn is weakened, and the lens, due to the elasticity of its capsule, takes on a shape closer to spherical. It should be noted that under the influence of multidirectional elastic forces: the lens capsule on one side and the choroid on the other, the anterior and posterior portions of the fibers of the ligament of Zinn in all phases of accommodation are in a constantly tense state of dynamic equilibrium. The degree of their tension during accommodation near is minimal, and during accommodation at a distance it is maximum. Cilioequatorial fibers do not participate in the process of accommodation and apparently serve to maintain the shape of the lens. The fact is that when accommodating near, the substance of the lens, tending to a spherical shape, has the smallest volume. When the lens stretches, its capsule stretches. The internal volume increases, and the intralens contents are incompressible. Cilioequatorial fibers stretch the capsule at the equator, preventing its possible “shrinking” or displacement of the intralens masses relative to the optical axis.
The description of the mechanism of accommodation also mentions the vitreous body. Its participation in providing optical adjustment of the eye for clear vision of objects at different distances is the subject of active debate. The main biomechanical role of the vitreous body, which fills the entire internal cavity of the eye behind the lens, is to ensure normal adhesion of the inner membranes of the eye to each other and to the sclera. In our opinion, the vitreous body directly, although passively, participates in the process of accommodation, damping and pressing the lens and allowing it to move slightly along the anterior-posterior axis of the eye.
Analysis of various theories of accommodation indicates that another factor determining the behavior of the eye during accommodation may be the stiffness of the sclera and IOP. However, calculations made using a biomechanical model show that the volume of accommodation is practically independent of the mechanical properties of the sclera if its longitudinal and circumferential elastic moduli are in the range from 5 MPa to 40 MPa. Although the ciliary muscle has an effect on the sclera, its surface moves slightly when the ciliary muscle contracts (the displacement does not exceed 0.02 mm). The influence of changes in IOP on the volume of accommodation has not been established if its value is from 10 to 30 mm Hg. Art. However, if the viscoelastic properties of the sclera are impaired (for example, with myopia), even a slight deformation of it with a relatively increased IOP can become partially irreversible and lead to a gradual change in the shape of the corneoscleral capsule of the eye and the progression of the myopic process.
Many accommodation specialists have expressed the opinion that extraocular muscles may play a certain role in the mechanism of accommodation, although most still consider them as additional participants in the accommodative process. An extreme point of view was expressed in this regard by NV Bates, who believed that the change in PZO caused by the pressure of the oblique muscles on the sclera is the main mechanism of accommodation, and the lens does not change shape at all.
To study the influence of extraocular muscles on accommodation, a series of calculations were performed within the framework of a biomechanical model of the eye, in which the contraction of the ciliary and different groups of extraocular muscles was simultaneously specified.
Modeling has shown that only excessive tone or mismatched contraction of extraocular muscles that unevenly compress the eyeball when rotating can have a significant impact on the volume of accommodation.
Such (mismatched) muscle action leads to deformation of the eyeball and to a slight increase in the length of its anteroposterior axis (APA). This is apparently explained by the asymmetrical attachment of the oculomotor muscles to the sclera and the uneven (in terms of the distribution of physical and mechanical properties) structure of the sclera, in particular, its increased rigidity at the tendon attachment points. The increase in the PPV is greater, the lower the modulus of elasticity of the sclera and the greater the mismatch of contractions of the extraocular muscles, but in the calculations it was not possible to obtain an extension of the PPV of more than 0.5 mm.
This means, in particular, that disruption of the balanced functioning of the extraocular muscles can lead to clinical consequences associated not only with strabismus, but also with impaired accommodative ability, as well as with the progression of myopia, since the mechanical stability of the myopic sclera is lower than normal (with emmetropia ).
Simulation of the work of extraocular muscles showed, however, that when they contract, there is a slight displacement of the center of the lens relative to the center of the cornea. The latter circumstance may explain the fact that during saccadic eye movements, visual perception is reduced.
This result coincides with the observations of Helmholtz: according to his theory, when viewing objects located at a close distance, in the human eye, along with contraction of the ciliary muscle, narrowing of the pupil, and a decrease in the depth of the anterior chamber, some displacement of the lens occurs anteriorly and downward.
Despite the practically absent normal effect on the value of POV, rotations of the eye (i.e. contractions of the extraocular muscles) to a certain extent cause deviation of the optical axis with a combination of lens accommodation and rotation of the eye or simply during rotation, which can lead to astigmatism as a compensatory reactions to deviation of the optical axis of the eye. In this case, the actual volume of accommodation changes slightly. Considering that normal deformations of the extraocular muscles do not exceed 15%, the maximum drop in the volume of accommodation (calculated value for 35 years) can be about 1 diopter. The short-term occurrence of image distortion associated with the work of the extraocular muscles most likely goes unnoticed. At the same time, long periods of distortion can lead to the need for their compensation, which in turn can cause the development of persistent astigmatism in the optical system of the eye.
The role of another structure of the eye - the cornea - in the mechanism of accommodation of the eye seems, according to the literature, controversial and not entirely unambiguous. According to Helmholtz's hypothesis, normally the cornea does not change its optical power and does not participate in the act of accommodation.