The “gold standard” for the treatment of certain diseases of the posterior segment of the eye is anti-VEGF therapy, namely the procedure of intravitreal administration of the drugs Eylea or Lucentis.
VEGF
(eng. vascular endothelial growth factor) - vascular endothelial growth factor.
Anti-VEGF
- antibodies to vascular endothelial growth factor.
The goal of anti-VEGF therapy is to reduce pathological neovascularization (vascular proliferation) and excessive permeability of newly formed vessels.
This goal is achieved by blocking growth factors of the endothelium of newly formed vessels, which, due to their incorrect structure, lead to recurrent hemorrhages and extensive swelling of the retina Retina
- the inner layer of the eye, an important part of the visual analyzer.
It is responsible for the process of converting light into a nerve impulse, which is transmitted to the brain. Such abnormal growth occurs due to the influence of various factors leading to the need to improve blood supply to the affected retinal tissue. The retina is the inner
lining of the eye, an important part of the visual analyzer. It is she who is responsible for the process of converting light into a nerve impulse, which is transmitted to the brain. through the formation of new blood vessels.
Data from clinical trials using anti-VEGF drugs
Systemic use as an intravenous infusion. Data are available from only one study of intravenous bevacizumab associated with ophthalmic pathology. This is the treatment of 18 patients suffering from neovascular AMD. In this uncontrolled study, the dose used was 5 mg/kg in 1, 2 and 3 injections, given at 2-week intervals. The visual acuity of patients during the study increased within two weeks from the start of drug administration and remained at the achieved level during 24 weeks of observation. By the end of the study, a significant decrease in retinal thickness was detected. At the same time, only six treated patients received additional therapy during the observation period. Despite the impressive results of the study, it was not designed to detect possible side effects.
Intravitreal injection. Quite large-scale clinical studies have been conducted on the use of pegaptanib and ranibizumab in patients with AMD. Pegaptanib was found to be less effective than ranibizumab. However, its use is associated with lower risks of undesirable consequences. Thus, according to the results of three central studies using ranibizumab, data were obtained on an increase in the incidence of cardiovascular disorders, including strokes and bleeding, although this increase was not statistically significant.
Several studies have reported positive results in treating patients with diabetes. In a double-blind, prospective, controlled, multicenter, dose-dependent study of 172 patients with diabetic macular edema, participants received pegaptanib and had a better prognosis for visual function at the end of the study (36 weeks). A decrease in central retinal thickness was found and fewer cases required additional laser treatment.
Currently, bevacizumab is used by many ophthalmologists around the world as preoperative therapy for proliferative DR before vitrectomy.
Anti-VEGF therapy
- VEGF
- VEGF-mediated pathology
- VEGF inhibitors
- Ranibizumab (Lucentis)
- Bevacizumab (Avastin)
- Brolucizumab (Beovu)
- Pegaptanib (Makugen, Macugen)
- Aflibercept (Aylia, Eylea, Eylea)
This treatment method is based on local inhibition of vascular endothelial growth factor (VEGF: vascular endothelium growth factor) when retinal neovascularization is detected (for example, in diabetic retinopathy (DME), wet AMD, as well as in post-thrombotic neovascularization), thereby stopping the growth of new fragile vessels with increased wall permeability. VEGF inhibitors may be an alternative or adjunct to conventional laser treatment and triamcinolone in patients with DME.
The development and introduction into practice of VEGF inhibitors for intravitreal administration have opened new prospects in the treatment of DME. The use of anti-VEGF therapy, either as a sole treatment method or in combination with LCS, reduces the severity of macular edema and improves visual acuity. Currently, ranibizumab (Lucentis, Genentech/Novartis) is registered in Russia for the treatment of DME. The indication for the use of this drug is macular edema with decreased vision and retinal thickness in the macular zone of more than 300 microns. However, positive results in randomized clinical trials were obtained with the use of bevacizumab, pegaptanib and aflibercept. Ongoing studies are planned to compare the effectiveness of these drugs in the treatment of DME (NCT01610557, NCT01627249).
Anti-VEGF drugs are administered intravitreally, as this is the most effective way to deliver the drug directly to the retina. With IVV, up to 51.4% remains in the vitreous body, and up to 13.2% of the administered dose penetrates into the retina and choroid. With other methods of administration - sujuconjunctival and sub-Tenon's - no more than 5.3% of the administered dose penetrates into the vitreous body, retina and choroid.
VEGF
VEGF was first isolated in 1983 as a factor that increases vascular permeability in tumors. VEGF belongs to a family of homodimeric glycoproteins, similar in structure to platelet growth factor, and binds to five types of receptors with tyrosine kinase activity. A variety of physiological and pathological processes associated with disturbances in the VEGF-VEGFR system include embryogenesis, regulation of reproductive function in women, pregnancy, wound healing, tumor growth, development of DR and ischemic diseases.
VEGF is a powerful multifunctional cytokine required for the processes of embryogenesis and early postnatal angiogenesis. In adults, VEGF in the vascular wall acts at different levels: as a factor ensuring the survival of endothelial cells, enhances vascular permeability and acts as a powerful vasodilator, is involved in muscle cell regeneration, myocardial remodeling and endochondral bone formation, it acts as a chemoattractant that mobilizes endothelial cells of the bone marrow . In addition to its physiological effects, VEGF has other effects that, although triggered by certain pathogenetic mechanisms, are beneficial. These include the ability to induce the formation of collateral circulation, which is necessary for cell survival under the influence of hypoxia, and improve trophism during wound healing.
The VEGF family in humans includes VEGF-A, -B, -C, -D, and placental growth factor (PlGF). Currently, the most studied is VEGF-A, which is expressed in many stromal and parenchymal cells and circulates in the bloodstream. In patients with diabetes mellitus (diabetes mellitus) with varying severity of angiopathy, an increase in the level of VEGF-A in the blood plasma and urine was recorded. Due to the blood-retinal barrier, the content of VEGF in the retina depends mainly on local production of the factor. Producers of VEGF in the retina are pigment epithelial cells, astrocytes, Müller cells, endothelial cells, pericytes and ganglion cells. Acting through auto- and paracrine pathways, VEGF selectively stimulates the proliferation and migration of endothelial cells and their precursors, increases vascular permeability, and promotes vasodilation through increased production of nitric oxide (NO).
In recent years, it has been shown that VEGF ensures the survival and structural integrity of the retinal pigment epithelium, has an anti-neurodegenerative effect and prevents apoptosis of retinal cells under ischemia-reperfusion conditions.
The molecular isoforms of VEGF (VEGF121, VEGF145,VEGF165, VEGF189, VEGF206) are products of a single gene, resulting from alternative splicing of mRNA. Polymorphic positions -634, +936, -2578 were identified in the VEGF gene; relationships between nucleotide variants in these positions and the risk of developing diabetic retinopathy (DR) were found in different ethnic groups.
Currently, hyperproduction of VEGF plays a leading role in increasing the permeability of retinal vessels, the development of macular edema and retinal neovascularization in diabetes. A powerful trigger for increased synthesis of VEGF and its receptors in DR is hypoxia or retinal ischemia. In addition, the production of VEGF in retinal cells is triggered by hyperglycemia and associated biochemical abnormalities: accumulation of late glycation products, endoplasmic reticulum stress, oxidative stress.
VEGF-mediated pathology
The retina is the most actively metabolizing human tissue; the intensity of metabolism and energy consumption per unit mass of tissue is much higher than that in all other tissues of the body. Complex biochemical processes accompanying the transformation of the energy of a light quantum when it is absorbed by photoreceptors into the energy of a bioelectric impulse and the subsequent transduction of this potential along bipolar and ganglion cells require constant provision of retinal tissue with adequate trophism.
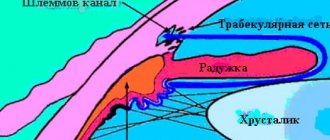
For this purpose, two collector vascular systems simultaneously exist in the human eye: the choroid (choroid), which supplies the outer parts of the retina, including photoreceptors, and the retinal vessels proper, which are responsible for the blood supply to the inner layers of the retina, including bipolar and ganglion cells. A specific anatomical feature of the retinal vessels is that they do not have collaterals. The consistency and normal functioning of both vascular systems is the basis for the normal functioning of retinal tissue.
In cases of disruption of the blood supply to any of the vascular collectors (regardless of the etiology and dynamics of development), a state of ischemia occurs in the retina, which is inevitably accompanied by a varying degree of loss of functional activity of the retina with the subsequent development of dystrophic and atrophic changes. Lack of oxygen triggers a cascade of pathological processes in the ischemic retina, many stages of which are mediated by a specific protein substrate—endothelial vascular growth factor.
VEGF is produced by endothelial cells, pericytes, and retinal pigment epithelial cells; it is present in the retina normally and regulates its homeostasis. In cases of ischemia, the production of VEGF in the retina increases significantly, which is the body's attempt to cope with ischemia by dilating existing vessels or growing new ones. In this case, the compensatory capabilities of the physiological mechanisms regulating the VEGE content are exceeded. As a result of dysregulation of the control of the content of vascular endothelial growth factor, its excessive secretion occurs, which leads to such functional and anatomical disorders of the normal vessels of the eyeball as:
- fenestration of vascular endothelium
- increased vascular permeability
- violations of the shape and morphological structure of blood vessels, etc.
At the macro level, such changes are accompanied by dysfunction of the choroidal or retinal vessels with the development of transudation and exudation (edema), hemorrhages. In a fairly short time, the next stage of the pathological process develops - the appearance of newly formed vessels or intraocular neovascularization, which can develop in various structures of the eye. A key role in the induction of neoangiogenesis belongs to the excessive concentration of VEGF in retinal tissue.
Depending on the source of ischemia (choroid or native retinal vessels), two types of intraocular neovascularization can be distinguished: choroidal and retinal. They are characterized by different morphological and clinical pictures and have specific features of development and progression. Common to all types of intraocular neovascularization is the inferiority of newly formed vessels, which consists in the absence of an endothelial basement membrane, as a result of which there are multiple fenestrae in the vascular wall, leading to transudation, exudation and hemorrhage. Similar changes are typical for any wound or inflammatory process when there is a violation of the integrity of the tissue.
Following the damage (alteration), an exudative reaction follows, and then the stage of scarring begins. The algorithm in the body is aimed at restoring the integrity of the tissue structure through intensive proliferation of connective tissue and is accompanied by partial or complete loss of specific tissue function. Scarring both on the surface of the retina, underneath it, and in the retina itself is inevitably accompanied by loss of vision function. Thus, the compensatory mechanism of intraocular neovascularization, intended to eliminate ischemia, becomes destructive from the point of view of the functional viability of the organ, another universal algorithm in the body aimed at restoring the integrity of the tissue structure through intensive proliferation of connective tissue and accompanied by partial or complete loss of specific tissue function. Scarring both on the surface of the retina, underneath it, and in the retina itself is inevitably accompanied by loss of visual function.
VEGF is not the only participant in the complex and diverse processes occurring in the retina under various ischemic conditions, however, today it is the most studied link in the pathogenesis, for which not only the role and place have been determined, but also methods of therapeutic influence and regulation have been developed that make it possible to effectively influence the pathological process and control the disease. Although the nosological forms in the pathogenesis of which VEGF is involved differ significantly, the presence of a common fundamental pathogenetic link in the form of VEGF allows us to combine all these diseases with the term “VEGF-mediated pathology.”
VEGF inhibitors
| VEGF inhibitors | ||||
| A drug | Tradename | Developer | Characteristic | Binding specificity |
| Ranibizumab | Lucentis | Genentech | Fragment of recombinant humanized antibodies to VEGF-A | Binds all VEGF-A isoforms |
| Bevacizumab | Avastin | Genentech | Recombinant humanized antibodies to VEGF-A | Binds all VEGF-A isoforms |
| Brolucizumab | Beovu | Novartis | Humanized single chain antibody fragment | Binds all VEGF-A isoforms |
| Pegaptanib | Macugen Eyetech | Pharmaceutical/Pfizer | PEGylated RNA aptamer | Binds VEGF165 |
| Aflibercept | Eylea VEGF Trap-Eye Regeneron | Pharmaceutical/Sanofi-Aventis | Recombinant antibody-like protein produced by fusing the supra-membrane portion of the VEGF receptor (as the antigen-binding fragment, Fab) and IgG (as the Fc fragment) | Binds VEGF-A, VEGF-B and PlGF |
| Bevasiranib | — | OPKO | Small interfering RNA | Inhibits the transcription of VEGF genes |
Differences between VEGF inhibitors relate to production technology, structure and specificity with respect to various isoforms of the regulator. The mechanism of action of anti-VEGF drugs is realized through direct binding to growth factors (ranibizumab, bevacizumab, pegaptanib), inhibition of the expression of the VEGF gene (bevasiranib) or its receptor (aflibercept). Currently, ranibizumab, bevacizumab, pegaptanib and aflibercept have undergone phase II–III clinical trials for DME.
Ranibizumab (Lucentis)
This VEGF inhibitor was specifically developed for use in ophthalmology. In 2006, the drug was approved by the Food and Drug Administration (FDA) for the treatment of neovascular (wet) age-related macular degeneration. Currently, it is the only anti-VEGF drug registered for the treatment of DME in Russia. The effectiveness of ranibizumab in the treatment of macular edema has been proven in a series of randomized multicenter studies performed according to GCP standards.
Bevacizumab (Avastin)
This drug is registered for use in metastatic colorectal cancer, breast cancer and some other malignant tumors; “off-label” is used in ophthalmology. More than 40 studies have examined bevacizumab in the treatment of DME [ClinicalTrial.gov].
New studies are currently being conducted comparing the effects of bevacizumab and the glucocorticoids triamcinolone, dexamethasone (NCT01572350, NCT01571232, NCT01787669), bevacizumab and ranibizumab (NCT01635790, NCT01627249) in the treatment of DME.
Brolucizumab (Beovu)
The brolucizumab molecule (RTH258) is a humanized single-chain antibody fragment capable of binding all isoforms of VEGF-A. Brolucizumab has the smallest molecular weight of 26 kDa, compared to 115 kDa for aflibercept and 48 kDa for ranibizumab (Gaudreault J. et al., Tietz J. et al.). Due to its low molecular weight, the concentration of brolucizumab when diluted can be increased to 120 mg/ml, that is, with one intravitreal injection it becomes possible to administer 6 mg of brolucizumab in 0.05 ml of solution. In terms of molecular weight, 6 mg of brolucizumab corresponds to approximately 12 doses of aflibercept 2 mg and 22 doses of ranibizumab 0.5 mg (Tietz J. et al.). Intravitreal administration of the drug in higher concentrations may potentially be accompanied by a longer duration of action.
Pegaptanib (Makugen, Macugen)
In the United States and Europe, the selective VEGF165 inhibitor pegaptanib is approved for the treatment of neovascular age-related macular degeneration. Several studies have assessed the drug's effect on DME. Analysis of retrospective data showed the ability of pegaptanib, when administered at a dose of 0.3 mg, to improve visual acuity in the first 6 weeks after injection. To obtain a significant effect, at least three injections were required within 6 months.
Aflibercept (Aylia, Eylea, Eylea)
The drug has the widest spectrum of anti-VEGF activity, binds VEGF-A, VEGF-B and PlGF. Approved in the US for the treatment of wet age-related macular degeneration and metastatic colorectal cancer. Studies of aflibercept (VEGF Trap-Eye) in DME began later than other anti-VEGF drugs.
Among the newest drugs that suppress the expression of VEGF, which are currently being studied for the treatment of MO, is bevasiranib (OPKO Health, USA). This is an RNA-based drug that is also administered intravitreally and suppresses the gene that produces VEGF.
"VEGF Trap-Eye" (Regeneron, USA and Bayer HealthCare, Germany) - a drug that was originally developed as an antitumor agent, is a "trap" for VEGF, since its molecule has a similar structure to the VEGF receptor. The drug is administered intravitreally and blocks all isoforms of VEGF-A, as well as PlGF12.
Ophthalmic complications of anti-VEGF therapy include endophthalmitis, increased intraocular pressure, lens damage, and retinal detachment. The frequency of these complications, according to most studies, does not exceed 1–1.5 cases per patient per year. Systemic adverse reactions are also rare and include increased blood pressure, stroke, myocardial infarction, and proteinuria. These reactions may be due to the penetration of small amounts of the drug into the bloodstream. Data were obtained that the level of VEGF in the bloodstream remains reduced for a month after intravitreal administration of bevacizumab and does not decrease after the administration of ranibizumab and pegaptanib. A study has now been initiated to evaluate the extent of leakage of VEGF inhibitors into the circulation following intravitreal administration (NCT01661946). It is required to specifically evaluate the safety of VEGF inhibitors in “problem” categories of patients with diabetes with macrovascular complications, severe arterial hypertension and nephropathy. Future studies will need to determine the optimal duration of anti-VEGF therapy, predictors of its effectiveness, and the advisability of combination with other methods of treating DME.
The use of antihypertensive drugs (1 drop at night for 14 days) to prevent an increase in IOP both in the early and late postoperative period helps prevent the occurrence of ocular hypertension, as well as improve microcirculation in the affected area.
Undesirable effects from the administration of VEGF inhibitors
Anti-VEGF drugs are injected into the vitreous directly through a puncture of the sclera, but their penetration into the systemic circulation is still possible. In turn, this can lead to undesirable systemic manifestations. At the same time, hypertension and proteinuria, which are detected especially often when the latter are used in the treatment of cancer, can be considered as markers of the systemic effect of anti-VEGF drugs. The increase in blood pressure is a consequence of an increase in the level of peripheral vascular resistance due to the suppression of the production of nitric oxide by endothelial cells, the formation of which is stimulated by VEGF through the activation of NO synthase, but is also explained by changes in renal function. Other potential complications that have been observed as a result of anti-VEGF use include suppression of muscle tissue regeneration with myocardial remodeling, infertility, changes in wound healing with the formation of collateral circulation, and gastrointestinal bleeding.
Thus, the potential systemic effects of the use of VEGF inhibitors (including hypertension, proteinuria, impaired wound healing, collateral circulation, etc.) can be dangerous, especially in individuals with diabetes mellitus.
Among the ophthalmic manifestations of anti-VEGF drugs, endophthalmitis, lens damage and retinal detachment are the most frequently reported. Serious complications arising in response to intraocular drug administration are quite rare. However, the cumulative risk is much higher in people with diabetes who require repeated treatment for many years.
In addition to side effects from the intravitreal injection itself, other potential undesirable effects arise, the development of which is due to the suppression of the action of VEGF. It should be noted that VEGF, formed by retinal pigment cells, ensures the functions of choriocapillaris and has a neuroprotective effect during retinal ischemia. Interestingly, when pegaptanib, which is unable to bind to VEGF120 (or human VEGF121), was used to suppress VEGF, there was no reduction in the number of retinal ganglion cells.
Very often, with intravitreal administration of bevacizumab (which blocks any known forms of VEGF), no toxic effect on retinal ganglion cells is observed. However, it is worth noting that to date there has been no evidence of its damaging effects on the retina, which could be detected by light microscopy. Nevertheless, mitochondrial destruction of the inner segments of photoreceptors (detected by electron microscopy), as well as increased apoptosis, were noted in an experiment on the eyes of rats after intravitreal administration of the drug.
To date, the development of VEGF inhibitors that suppress the pathological effects of VEGF while maintaining neuroprotective effects continues, and this will likely be a significant breakthrough in ensuring the safety of new drugs.
VEGF inhibitors
VEGF inhibitors differ in some aspects associated with their preparation, as well as details of the structure of the substances and specificity in relation to various isoforms of the regulator. The effect of anti-VEGF drugs (ranibizumab, bevacizumab, pegaptanib) is due to direct binding to growth factor, suppression of the expression of the VEGF gene or its receptor. To date, phase II–III clinical trials of AMD therapy have been completed: bevacizumab, pegaptanib, ranibizumab, aflibercept. Most of them are already officially approved as treatments for age-related macular degeneration.
Ranibizumab (Lucentis)
The substance contained in the drug Lucentis was specifically addressed to ophthalmologists. This VEGF inhibitor has been approved by the Food and Drug Administration (FDA) for use in the United States for the treatment of age-related wet macular degeneration. Today, this is the only drug with anti-VEGF action officially registered in Russia. Its effectiveness in the treatment of macular edema has been proven by a series of randomized studies conducted in many ophthalmology centers.
Bevacizumab (Avastin)
This anti-VEGF drug is mainly used in oncology for the treatment of malignant tumors; in ophthalmology its use does not have an official status (“off-label”). To date, more than 40 clinical studies have focused on the effectiveness of bevacizumab in the treatment of wet AMD. Moreover, the latest of them were aimed at comparing the effect of bevacizumab and corticoids (triamcinol, dexamethasone) in the treatment of neovascular macular degeneration.
Pegaptanib (Makugen, Macugen)
This anti-VEGF drug is approved for the treatment of neovascular AMD in the United States and European countries. Its effect has been confirmed by a number of studies. According to the data obtained, pegaptanib administered at a dose of 0.3 mg significantly improves visual acuity, the result lasting 6 weeks after injection. A long-term effect is achieved by administering at least three injections over six months.
Aflibercept (Aylia, Eylea, Eylea)
This drug has the broadest anti-VEGF activity. It is able to act on VEGF-A, VEGF-B and PlGF. In the USA, it is approved for use in the treatment of wet neovascular macular degeneration, as well as metastatic colorectal tumors. Research on aflibercept as a treatment for AMD began later than other anti-VEGF drugs.
Among the latest generation of anti-VEGF agents that are just undergoing the necessary studies for subsequent use as a treatment for macular edema, it is worth noting bevasiranib. This drug, which is based on RNA, suppresses the gene that synthesizes VEGF. It is also administered intravitreally.
The drug “VEGF Trap-Eye” is already quite well known. It was originally developed to fight tumors, but became a real “trap” for VEGF, because its molecular structure is similar in shape to the VEGF receptor. When administered intravitreally, the drug neutralizes all VEGF-A and PlGF12 isoforms.
What is anti-VEGF treatment?
Anti-VEGF drugs work in the body by blocking the production of endothelial vascular growth protein, which stimulates the production of blood vessels when the body needs it.
The VEGF protein increases its presence in the body when a person suffers from certain eye diseases associated with the appearance of new blood vessels (neovas).
Sometimes cells can produce a lot of VEGF protein, and when this happens, there is an increased risk of abnormal blood vessels in the body. ocular structure.
Abnormal blood vessels can significantly impair vision and vision, leading to blindness.
What diseases does anti-VEGF treat?
Anti-VEGF treatment is prescribed primarily for the treatment of DMAE wet and diabetic retinopathy. Both diseases are considered important causes of blindness in the world.
- La DMAE is a vascular disease that affects the central part of the retina, which is responsible for central vision and the perception of details. Wet AMD is the least common form of macular degeneration, but it is the most aggressive and therefore requires action quickly and with the right treatment to stop vision loss.
- La diabetic retinopathy This is a condition that occurs due to deterioration of the blood vessels supplying the retina. When blood vessels are damaged, there is a risk of blood fluid leaking or spilling. Diabetic retinopathy is caused by high levels of glucose in the blood.
Pathogenesis of diabetic retinopathy
Chronic hyperglycemia plays an important role in the development of diabetic retinopathy. As a result of this, a large number of biochemical, molecular genetic, and pathophysiological mechanisms are triggered. All this affects the retinal vessels and leads to damage to the endothelium. In this case, the retinal vascular bed becomes empty, the wall becomes more permeable, which causes plasma hyperfiltration, hemorrhages, and retinal hypoxia.
In response to a lack of oxygen, retinal cells actively produce a specific protein that increases transcription of the VEGF gene. This substance affects the epithelium, stimulates the proliferation and regeneration of blood vessels.
Vascular endothelial growth factor in normal and pathological conditions
The VEGF protein was first isolated back in 1983. Its main goal was to increase the permeability of the vascular wall of the arteries located in the tumor node. This protein belongs to the glycoproteins and has structural similarities to platelet growth factor. It contains five types of receptors (with tyrosine kinase activity), that is, VEGF can be influenced by the processes of embryogenesis, pregnancy, wound healing, tumor growth, reproductive function in women, the development of coronary disease and diabetic retinopathy.
The VEGF-A protein has been studied to the greatest extent. This substance has several isoforms, but its main effect on cells is through the tyrosine receptor, which is located in the membrane structure. With intravitreal administration of the VEGF 165 blocker, a significant slowdown in the processes of pathological neovascularization occurs, while the effect on physiological vascularization was practically absent or insignificant.
In addition, VEGF is involved in embryogenesis and early angiogenesis. In adults, endothelial growth factor acts in two ways in the vascular wall: on the one hand, it promotes the survival of endothelial cells, on the other, it stimulates vascular permeability and causes dilation of the lumen of the arteries. For example, in the kidneys, VEGF controls the functioning of the filtration system and is responsible for glomerulogenesis. In addition, this protein affects the regeneration of muscle cells, the restructuring of the myocardium and causes the formation of bone tissue inside the cartilage.
In addition to physiological processes, VEGF leads to the launch of several other beneficial mechanisms. These include the growth of collateral vessels in response to hypoxia, as well as improved tissue nutrition during wound healing.
In patients with diabetes, VEGF is secreted by retinal pigment epithelial cells, resulting in the growth of new blood vessels and retinal edema. Type 1 diabetes is characterized by neovascularization processes, while type 2 diabetes is more likely to cause retinal edema, which leads to loss of central vision.