Aqueous humor circulates along the episcleral and intrascleral venous network of the anterior segmented area of the eyeball. It supports the metabolic processes of the cornea, lens, and trabecular apparatus. Under normal circumstances, the human eye contains 300 mm of the component or 4% of the total volume.
The fluid is produced from the blood by special cells that are part of the structure of the ciliary body. The human eye produces 3-9 ml of the component per minute. The outflow of moisture occurs through the episcleral vessels, uveoscleral system and trabecular meshwork. Intraocular pressure is the ratio of the produced component to the withdrawn component.
Structure
Functions
Symptoms
Treatment
Anatomical functions
Aqueous humor contains immunoglobulins, glucose and amino acids, which strengthen and nourish the lens, the anterior part of the vitreous body, the corneal endothelium and other non-vascularized structures of the eye. The presence of immunoglobulins in the aqueous humor and constant circulation help remove potential damage factors from the inside of the eye.
Aqueous humor contains less urea and glucose compared to plasma, since most of the plasma is processed by the lens. The moisture content does not include > 0.02% protein, a proportion of creatine, riboflavin, hexosamine, hyaluronic acid and other chemical compounds. Domestic scientists believe that it is the aqueous humor that controls the constant pH level through the deep processing of metabolic products of intraocular tissues.
Diseases associated with lack of aqueous humor
Maintaining a normal volume of aqueous humor is an important task for the ophthalmologist during surgical interventions. Loss of some moisture during operations or injuries can further cause hypotony of the eye. In such cases, it is important to contact an ophthalmology clinic as soon as possible to compensate for the normal level of IOP and restore the volume of aqueous humor.
Also, disturbances in the outflow of aqueous humor cause an increase in IOP and, as a rule, the development of glaucoma.
Intraocular aqueous fluid is colorless. This is a transparent substance that is similar in composition to blood plasma. Unlike the latter, it contains less protein. Aqueous humor is found in both eye chambers. The fluid is formed by special cells of the ciliary body of the eye. These cells produce moisture by filtering the blood. Up to 9 ml of liquid can be generated per day.
Circulation of intraocular fluid
The secreted fluid enters the posterior ocular chamber. Through the opening of the pupil it enters the anterior chamber of the eye. Under the influence of temperature changes, moisture flows through the iris into the upper layers, after which it flows down along the inner surface of the cornea. The water then enters the angle of the anterior chamber of the eye, where it is absorbed through the trabecular meshwork into Schlemm's canal. The final stage of the chain is the entry of aqueous humor of the eye with metabolic products back into the bloodstream.
What is the function of aqueous humor?
The intraocular fluid is rich in amino acids, glucose and other nutrients. It provides the eye structures with useful substances. In particular, the liquid nourishes tissues that lack blood vessels - the lens, trabecula, and the anterior part of the vitreous. In addition, aqueous humor prevents the development of pathogens due to the immunoglobulins it contains.
In addition, the intraocular fluid is another transparent medium that refracts light. It provides the shape of the eye, the value of intraocular pressure (IOP) depends on it. The latter is precisely the balance between the amount of moisture produced and that leaving the bloodstream.
Symptoms of disorders of the outflow of intraocular fluid
Normal circulation of aqueous humor ensures IOP within the range of 18-25 mm Hg. s.t. If production or outflow is impaired, pressure may decrease (hypotension) or increase (hypertonicity). In the first case, retinal detachment may occur. As a result, vision decreases until it is completely lost. With increased eye pressure, the patient feels pain in the head, impaired vision and nausea. If the disease is not treated, inevitable destruction of the optic nerve and loss of vision occurs.
Diagnosis of disorders
- Visual examination, palpation of the eye.
Ophthalmoscopy.
Tonometry.
Capimetry.
Perimetry.
High intraocular pressure and glaucoma
With increased production or difficulty in the outflow of aqueous humor from the eye, intraocular pressure increases, which leads to glaucoma. This destroys the fibers of the optic nerve. As a result, visual acuity decreases up to complete blindness. The risk of increased pressure inside the eye is significantly higher in people over forty years of age. The danger of glaucoma lies in the absence of unpleasant symptoms, which is why the disease remains hidden to the patient for a long time, although it progresses. To diagnose glaucoma in a timely manner, patients over 40 years of age need to have their intraocular pressure checked at least once a year.
So, the intraocular fluid ensures the normal functioning of the entire eyeball. The pressure in the anterior and posterior chambers of the eye depends on it. Unfortunately, serious pathological changes can occur from disruption of the production or outflow of fluid in the eye. Increased intraocular pressure inevitably causes glaucoma. To avoid irreversible disturbances in the functioning of the visual apparatus, ophthalmologists recommend regularly checking intraocular pressure.
Methods for removing foreign bodies from the conjunctival sac and cornea:
1) foreign bodies located in the superficial layers of the cornea sometimes fall out on their own
2) to remove superficially located foreign bodies, in addition to ordinary needles, flat and grooved chisels, tweezers, a dental bur, etc. are used.
3) to remove it from the corneal stroma under local anesthesia, an incision is made into the cornea with a linear knife or razor blade above the location of the fragment, then a magnet is used. If the foreign body cannot be removed with a magnet, it is removed with a spear or needle.
4) after epibulbar anesthesia with 0.5% dicaine solution, foreign bodies of the conjunctiva are removed with a damp swab or a small injection needle.
Prevention of eye injuries:
a) strict adherence to technical and safety rules and compliance with sanitary and hygienic standards in production premises, purification of air in enterprises from smoke, dust, vapors, good lighting
b) individual eye protection with goggles and masks; use of protective devices on working machines.
c) combating childhood injuries among teachers, parents, public organizations
Ticket No. 16
Glaucoma
- Classification
- Etiology
- Pathogenesis
- Clinical picture of primary glaucoma
- Screening
- Treatment
- Agents that improve the outflow of intraocular fluid
- Drugs that inhibit the production of intraocular fluid
- Absolute glaucoma
Glaucoma is a genetically heterogeneous group of visual neuropathies that cause irreversible visual field defects. Glaucoma is inherited both as a monogenic and as a complex disease, which is often associated with increased intraocular pressure (IOP), defects in the trabecular meshwork and anterior chamber of the eye, leading to impaired outflow of intraocular fluid, and progressive degeneration of the optic nerve. However, elevated IOP is neither necessary nor sufficient for the onset or progression of the disease. IOP-independent mechanisms are also involved in glaucomatous degeneration.
- There are 1,336,508 patients with glaucoma in the Russian Federation (2018)
- Of these, patients diagnosed for the first time in life, 126,380 (2018)
- There are 1,086,752 patients with glaucoma under dispensary observation (2018)
- Over 70,000 people are blind due to glaucoma. Over the past five years, there has been an increase in primary disability due to glaucoma from 22.1 to 28.8%.
- In almost all regions of Russia, glaucoma ranks 1st among the causes of disability due to ophthalmopathology - 29%.
Classification
Glaucoma is classified by etiology (primary and secondary), anterior chamber anatomy (open-angle and closed-angle) and time of onset (infantile-juvenile and adult). Various forms of primary glaucoma are divided into 3 main groups: open-angle (POAG) - the most common, acute angle-closure, congenital (hereditary) glaucoma.
Glaucoma is divided into:
- by origin – primary, secondary and combined with developmental defects of the eye or other organs; Glaucoma may be associated with other congenital anomalies, including microcornea, sclerocornea, aniridia, persistent primary vitreous, peripheral or neutral mesodermal dysgenesis (Rieger syndrome, Frank-Kamenetsky syndrome, Peters anomaly), homocysteinuria, Marfan syndrome, Marchesani syndrome, Lowe syndrome, Sturge-Weber fibromatosis , neurofibromatosis, chromosomal disorders.
- according to the patient’s age – congenital (up to 3 years). Caused by defects in the development of the anterior chamber angle or drainage system of the eye. The disease manifests itself in the first three years of a child’s life; heredity is recessive (sporadic cases are also possible). The pathogenesis of the disease is based on dysgenesis of the anterior chamber angle and increased IOP. Clinical symptoms are varied: photophobia, lacrimation, blepharospasm, enlargement of the eye, corneal edema and increase in its size, atrophy of the optic disc with excavation.
- infantile, (from 3 to 10 years). Heredity and pathogenesis are the same as with congenital glaucoma, IOP is increased, the size of the cornea and eye is not changed, excavation of the optic disc increases as glaucoma progresses.
- juvenile (from 11 to 35 years). Heredity is associated with disorders of both chromosome I and TIGR. Trabeculopathy and/or goniodysgenesis play a leading role in the pathogenesis of the disease. The IOP level is increased, changes in the optic disc and visual functions occur according to the glaucomatous type.
- adult glaucoma; (over 40 years old) - Includes primary open-angle, closed-angle and secondary glaucoma. Primary open-angle glaucoma is in turn divided into primary, pseudoexfoliative, pigmentary, and normal-tension glaucoma.
- to open-angle (primary, complicated by pseudoexfoliation syndrome, complicated by pigment dispersion syndrome),
- Normal - P1 - up to 25 mmHg, P0 - up to 21 mmHg,
- I - initial, - the boundaries of the visual field are normal, but there are small changes (scotomas) in the paracentral regions. The excavation of the optic nerve head is expanded, but does not reach its edge.
- stabilized - with long-term observation of the patient (at least 6 months), no deterioration in the optic disc or visual field condition is detected.
The first classification of glaucoma, proposed by A. Graefe (1857), was based on clinical symptoms. Graefe distinguished between acute inflammatory and chronic glaucoma and identified four stages of the disease:
- prodromal,
- developed,
- absolute and
- degenerative.
He also described amaurosis with excavation of the optic disc as an independent disease, which Donders (1862) later called simple glaucoma. Thus, back in the last century, there was an idea of two main forms of glaucoma: inflammatory (stagnant, congestive) and simple.
A new step towards the creation of a pathogenetic classification of glaucoma was made by O. Barkan (1938,1954). which, based on the results of gonioscopy. identified two types of glaucoma - narrow-angle and wide-angle. Later it was found that in the pathogenesis of glaucoma, angle narrowing does not matter until it closes completely.
In this regard, Barkan's classification was slightly changed: glaucoma was divided into closed-angle and open-angle. Gonioscopic classification of glaucoma has gradually replaced clinical classifications, and is now universally accepted.
The original version of the pathogenetic classification of glaucoma was proposed by M. M. Krasnov (1965). The author distinguishes hypersecretory and retention forms of primary glaucoma.
Retention glaucoma, associated with difficulty in the outflow of aqueous humor from the eye, can be of three types: angular, trabecular and intrascleral. Krasnov points out the possibility of combined forms of retention glaucoma: a combination of angular glaucoma with trabecular, trabecular with intrascleral.
S.N. Fedorov identifies three types of primary open-angle glaucoma:
- ciliary - the lesion is localized in the basin of the posterior long and anterior ciliary arteries. In such cases, atrophy of the iris and ciliary body, increased IOP, a gradual decrease in visual function and atrophy of the optic nerve head are observed.
- ciliouveal - is a consequence of damage to the posterior short and long ciliary arteries. The symptoms are the same, but changes in the choroid are added, and atrophy of the optic nerve head progresses even after normalization of IOP.
- papillary - the arteriocapillary network supplying the optic nerve head is affected, IOP remains normal, but atrophy of the optic nerve head quickly develops.
A new approach to creating a classification of glaucoma was used by B. L. Polyak (1952), who proposed dividing the glaucomatous process into stages (according to the state of the visual field and optic nerve head) while simultaneously dividing it into three groups depending on the state of compensation. The main advantage of the Polyak classification is that it can be used to make a prognosis of the disease and monitor the dynamics of the glaucomatous process, which is especially important during dispensary observation of the patient.
For more than 20 years, this classification has been generally recognized in our country. In connection with significant progress in the study of the pathogenesis of primary glaucoma and its various forms, the question was raised about creating a new classification, which was developed on the instructions of the All-Union Scientific Society of Ophthalmologists A. P. Nesterov and A. Ya. Bunin (1977).
Etiology
Glaucoma is a multifactorial disease that has a threshold effect. In other words, for the development of this pathology, a number of reasons are necessary that will determine its occurrence. The most important factors are:
- Genetic predisposition;
- Individual anomalies in the structure of the eye;
- Pathologies of the nervous, cardiovascular or endocrine systems.
Today, scientists have put forward the assumption that the development and progression of the pathological process in question represents a sequential chain of etiological factors, the action of which is summed up and triggers the process that leads to the onset of the disease. Despite this, the mechanisms of visual dysfunction in the morphology of glaucoma remain insufficiently studied to date.
Pathogenesis
The main stages of development of the pathological process occurring in glaucoma can be presented as follows:
- There is a deterioration in the outflow of intraocular fluid from the cavity of the eyeball;
- Intraocular pressure exceeds the tolerable level for the eye in question;
- Blood circulation within the eye tissues deteriorates;
- Tissues in the area where the optic nerve exits are subject to ischemia (impaired blood supply) and hypoxia (lack of oxygen);
- Compression of the nerve fibers occurs in the area where they exit the eyeball, which causes disruption of their function and death;
- Dystrophy, destruction and atrophy of the optic fibers develop, and their maternal retinal ganglion cells disintegrate;
- Glaucomatous optic neuropathy occurs, which leads to optic nerve atrophy.
Depending on the stage of the glaucomatous process, some of the nerve fibers of the optic nerve die, and the other part goes into a state of parabiosis (a kind of “sleep”), which allows doctors to consider restoration of their function possible.
Clinical picture of primary glaucoma
Open-angle glaucoma is a genetically determined disease, the likelihood of developing which increases if one or more of the following factors are present:
- Heredity;
- Elderly age;
- Myopia;
- General diseases (arterial hypertension and/or hypotension, diabetes mellitus, cervical osteochondrosis, atherosclerosis, etc.).
There is an assumption that these factors cause a deterioration in the blood supply to the eyes and brain, which leads to disruption of normal metabolic processes in the eye.
As a rule, open-angle glaucoma occurs and develops almost unnoticed by the patient. The patient does not experience absolutely any unpleasant sensations, seeking medical help already at the moment when the pathological process reaches late stages and vision deterioration is noted. Only 15-20% of patients note periodic blurred vision and the appearance of rainbow circles near light sources. It is these symptomatic manifestations that occur with increased intraocular pressure. There may also be pain localized in the eyebrow area and the head as a whole.
In most cases, open-angle glaucoma affects both eyes, occurring asymmetrically.
The leading symptomatic manifestation of the disease is increased intraocular pressure. This process occurs rather slowly and gradually, as resistance to the outflow of intraocular fluid increases. Initially it is periodic in nature, then it becomes permanent.
The most important diagnostic sign of the disease in question is a change in the visual field. First of all, these defects arise in the central sections, manifested by the appearance of arcuate prolapses and expansion of the boundaries of the blind spot. Such disorders are diagnosed in the early stages of the disease during special visual field studies. In most cases, patients themselves do not notice these changes.
With further progression of the glaucomatous process, defects in the peripheral visual field are detected. Predominantly, the narrowing of the field of vision occurs on the nasal side, subsequently concentrically covering all peripheral parts (up to complete loss of vision).
Dark adaptation deteriorates. All these symptoms are observed against the background of a persistent increase in intraocular pressure. A decrease in visual acuity indicates an advanced stage of the disease, in which almost complete atrophy of the optic nerve occurs.
Angle-closure glaucoma is a form of the disease that accounts for 20-25% of cases of primary glaucoma. Women get sick more often than men. The risk of developing this form of glaucoma increases with the presence of the following factors:
- Anatomical predisposition (small eye size, large lens, small anterior chamber, farsightedness, narrow anterior chamber angle);
- Age-related changes in the eye;
- Functional factors for closing the anterior chamber angle (increased level of intraocular fluid production, pupil dilation, increased blood supply to intraocular vessels).
The development of angle-closure glaucoma in the vast majority of cases is characterized by periodic – initially short-term, with constantly increasing duration – periods of increased intraocular pressure. At the initial stage of the disease, this is due to the fact that the trabecular zone is mechanically closed by the root of the iris - this is a consequence of the anatomical predispositions of the eye. This reduces the outflow of intraocular fluid. After complete closure of the anterior chamber angle, the patient experiences a condition called an acute attack of angle-closure glaucoma. In the intervals between attacks, the angle opens.
During such attacks, a gradual formation of adhesions occurs between the wall of the anterior chamber angle and the iris. Over time, the pathology becomes chronic, accompanied by a constant increase in intraocular pressure.
In the clinical picture of angle-closure glaucoma, the following phases can be distinguished:
- Preglaucoma;
- Acute attack of glaucoma;
- Chronic glaucoma.
Preglaucoma affects individuals who have no clinical manifestations of the disease, but a detailed examination of the anterior chamber angle reveals that it is either closed or narrow. During the period of time between preglaucoma and an acute attack of the disease, transient symptoms of visual discomfort, short-term loss of vision, and the appearance of rainbow circles when looking at a bright light source may occur. Most often, such manifestations occur during emotional arousal or during a long stay in low light conditions (such conditions cause pupil dilation, which partially or completely reduces the outflow of intraocular fluid) and, as a rule, disappear on their own. In most cases, patients pay little attention to these symptoms.
An acute attack of glaucoma is the result of the influence of provoking factors:
- Nervous tension;
- Prolonged stay in the dark;
- Overwork;
- Prolonged work in a position with the head tilted;
- Medicinal dilation of the pupil;
- Taking large amounts of liquid.
In some cases, an attack occurs for no apparent reason. The patient complains of pain in the eye and head, the appearance of rainbow circles when a light source enters the field of view, blurred vision. The pain is a consequence of compression of the nerve elements in the ciliary body and the root of the iris. Visual discomfort is caused by corneal edema. A severe attack may be accompanied by vomiting and nausea, in some cases by pain that radiates to the abdomen and heart (very similar to manifestations of cardiovascular pathologies).
During a visual examination of such an eye, performed without special instruments, one can only see a sharp dilation of the vessels located on the front surface of the eye. The eyeball becomes “red” with some bluish tint (a sign of congestive vascular injection). Due to the development of edema, the cornea becomes somewhat cloudy. A characteristic sign is also a dilated pupil that does not respond to light. Another manifestation of an acute attack of glaucoma can be a sharp decrease in visual acuity. Intraocular pressure can rise to 80 mm Hg. Art., while the outflow of fluid from the eye practically stops. The eye becomes as dense as stone to the touch.
If the pressure is not reduced within several hours after the attack occurs (by surgery or through the use of medications), the eye may permanently lose vision. An acute attack of glaucoma is an emergency and requires immediate medical attention.
Over time, this pathology becomes chronic. This form of glaucoma occurs with a progressive increase in intraocular pressure, increasing blockade of the anterior chamber angle and subacute attacks. The processes under consideration naturally lead to the development of glaucomatous optic nerve atrophy and loss of visual functions.
Screening
- The classic triad of symptoms, any of which suggests glaucoma in newborns and young children, includes lacrimation, photophobia, and blepharospasm.
- Any patient over 35 years of age who consults an ophthalmologist should undergo a general ophthalmological examination.
- Patients over 50 years of age should have IOP measured and the fundus examined during routine medical examinations (at least once a year).
- Angle-closure glaucoma should be suspected and a visual examination should be performed in patients with systematic complaints of headache, nausea and vomiting. The examination includes determination of visual acuity, IOP, biomicroscopy, ophthalmoscopy and perimetry.
- It is necessary to remember the likelihood of increased IOP in patients taking corticosteroids (dexamethasone, prednisolone, etc.), anticholinergic agents (atropine, methocinium iodide, pirenzepine, ipratropium bromide) or adrenergic agonists (salbutamol, formoterol, terbutaline).
- If glaucoma is suspected, it is necessary to perform at least a rough assessment of the anterior chamber angle (hand-torch test or Van Herrick test using a slit lamp). If tests with a hand-held flashlight or slit-lamp illuminator are positive (the light reflex on the sclera is wide and clearly visible), then there is no likelihood of closure of the UPC. If the tests are negative, gonioscopy is mandatory.
Treatment
Nerve damage and vision loss from glaucoma cannot be reversed, but treatments are available to slow or stop the progression of the disease. Treatment can normalize intraocular pressure and prevent or stop further nerve damage and blindness.
Treatment may include the use of eye drops, tablets (rarely), laser and other methods, or microsurgery to treat glaucoma.
Treatment regimen for glaucoma with elevated IOP levels
- Prescribing monotherapy with the drug of first choice (prostaglandins)
- Change of monotherapy drug
- Combination with first or second line drugs
- Laser treatment
- Surgery
- Additional drug treatment
Treatment regimen for normal pressure glaucoma
- Purpose of prostaglandins
- Combination of prostaglandins and β-blockers
- Addition of second-line drugs, primarily carbonic anhydrase inhibitors (brinzolamide 1%, dorzolamide 2%)
- Early surgical treatment
Agents that improve the outflow of intraocular fluid
- Miotics (pilocarpine and carbachol)
- Sympathomimetics
- Epinephrine - Glaucon 1% and 2%, epiphrine 0.5%, 1% and 2%
- Dipivefrin - Oftan-dipivefrin - 0.1%
- Prostaglandins F2 alpha
- Latanoprost (Xalatan 0.005%) is a PGF2alpha analogue, a selective agonist of prostanoid FP receptors
- Travoprost (Travatan 0.004%) is a synthetic analogue of PGF2α., is a highly selective full agonist of prostaglandin FR receptors and reduces IOP by increasing the outflow of aqueous humor through the trabecular meshwork and uveoscleral pathways.
- Tafluprost (Taflotan 0.0015%) is a fluorinated analogue of prostaglandin F2α.
Drugs that inhibit the production of intraocular fluid
- Selective sympathomimetics - Clonidine (clonidine) - Clonidine 1.125%, 0.25%, 0.5%
- Beta blockers
- Non-selective (ß1.2) adrenergic blockers - Timolol 0.25%, 0.5% - Oftan timolol, Timolol-LENS, Timolol-DIA, Timohexal, Arutimol, Kusimolol, Niolol, Okumed, Okumol, Timoptic, Timoptic-depot - prolonged form .
- Selective (ß1) adrenergic blockers. Betaxolol 0.5% - Betoptik 0.5%, Betoptik S 0.25% ophthalmic suspension
- Carbonic anhydrase inhibitors - selectively inhibit the activity of carbonic anhydrase II (CA-II) - Dorzolamide (Trusopt 2%) and Brinzolamide (Azopt 1%)
Combination drugs
- Proxofelin (proxodolol + clonidine),
- Fotil (timolol 0.5% + pilocarpine 2%),
- Fotil forte (timolol 0.5% + pilocarpine 4%),
- Normoglaucon (pilocarpine 2% + metypranolol),
- Cosopt (dorzolamide 2% + timolol 0.5%)
Absolute glaucoma
It is the final stage of all types of glaucoma. In this case, the eye is completely blind (no light perception), has a rocky density, and is characterized by severe pain. For absolute glaucoma with extremely severe pain, the following are used: retrobulbar administration of alcohol (or chlorpromazine), radiotherapy, neurectomy, eye removal (rarely).
Cameras of the eye. Pathways for the outflow of intraocular fluid.
Front camera
is a space bounded by the posterior surface of the cornea, the anterior surface of the iris and the central part of the anterior capsule of the lens. The place where the cornea meets the sclera and the iris meets the ciliary body is called the anterior chamber angle. The anterior chamber angle is the narrowest part of the anterior chamber. The anterior wall of the AC is the Schwalbe ring, the trabecular apparatus and the scleral spur, the posterior wall of the AC is the root of the iris, the apex is the base of the ciliary crown. On the outer wall of the UPC there is the drainage system of the eye.
The drainage system of the eye consists of the trabecular apparatus, scleral sinus (Schlemm's canal) and collector tubules. The trabecular apparatus is a ring-shaped crossbar thrown across the internal scleral groove. On a section, it has the shape of a triangle, the apex of which is attached to the anterior edge of the groove (Schwalbe's boundary ring), and the base to its posterior edge (scleral spur). The trabecular diaphragm consists of three main parts: the uveal trabecula, the corneoscleral trabecula, and the juxtacanalicular tissue. The first two parts have a layered structure. Each layer (there are 10-15 in total) is a plate consisting of collagen fibrils and elastic fibers, covered on both sides with a basement membrane and endothelium. There are holes in the plates, and between the plates there are gaps filled with liquid fluid. The juxtacanalicular layer, consisting of 2-3 layers of fibrocytes and loose fibrous tissue, provides the greatest resistance to the outflow of fluid from the eye. The outer surface of the juxtacanalicular layer is covered with endothelium containing giant vacuoles. The latter are dynamic intracellular tubules through which the fluid passes from the trabecular apparatus to Schlemm's canal.
Schlemm's canal is a circular fissure lined with endothelium and located in the posterolateral part of the internal scleral groove. It is separated from the anterior chamber by the trabecular apparatus; outward from the canal there is the sclera and episclera with venous and arterial vessels. The fluid flows from Schlemm's canal through 20-30 collector tubules into the episcleral veins (recipient veins).
Through the pupil, the anterior chamber freely communicates with the posterior one. Rear camera
is located behind the iris, which is its anterior wall and is bounded externally by the ciliary body and behind by the vitreous body. The inner wall is formed by the equator of the lens. The entire space of the posterior chamber is penetrated by ligaments of the ciliary girdle.
Normally, both chambers of the eye are filled with aqueous humor, which in its composition resembles blood plasma dialysate. Aqueous humor contains nutrients (glucose, ascorbic acid, oxygen) used by the lens and cornea, and removes metabolic products (lactic acid, carbon dioxide, exfoliated pigment and other cells) from the eye.
Production and outflow of intraocular fluid (IoF).
The fluid is continuously produced by the ciliary crown with the active participation of the non-pigmented retinal epithelium and, in smaller quantities, in the process of ultrafiltration of the capillary network. Moisture fills the posterior chamber, then enters the anterior chamber through the pupil (it serves as its main reservoir and has twice the volume than the posterior one) and flows mainly into the episcleral veins through the drainage system of the eye, located on the anterior wall of the anterior chamber angle. About 15% of the fluid leaves the eye, seeping through the stroma of the ciliary body and sclera into the uveal and scleral veins - the uveoscleral outflow tract of the fluid. A small part of the liquid is absorbed by the iris (like a sponge) and the lymphatic system.
Regulation of intraocular pressure. The formation of aqueous humor is under the control of the hypothalamus. A certain influence on secretory processes is exerted by changes in pressure and the rate of blood outflow in the vessels of the ciliary body. The outflow of intraocular fluid is regulated by the ciliary muscle - scleral spur - trabecula mechanism. The longitudinal and radial fibers of the ciliary muscle are attached to the scleral spur and trabecula with their anterior ends. When it contracts, the spur and trabecula move posteriorly and inwardly. The tension of the trabecular apparatus increases, and the openings in it and the scleral sinus expand.
Front camera
(camera anterior) - a space limited in front by the cornea, behind by the iris and in the area of the pupil by the lens.
The depth of the anterior chamber is variable, it is greatest in the central part of the anterior chamber, located opposite the pupil, and reaches 3-3.5 mm. Under pathological conditions, both the depth of the chamber and its unevenness acquire diagnostic significance. The posterior chamber
is located behind the iris, which is its anterior wall.
The outer wall is the ciliary body, the back wall is the anterior surface of the vitreous body. The inner wall is formed by the equator of the lens and the pre-equatorial zones of the anterior and posterior surfaces of the lens. The entire space of the posterior chamber is permeated with fibrils of the ligament of zinn, which support the lens in a suspended state and connect it to the ciliary body. The chambers of the eye are filled with aqueous humor - a transparent, colorless liquid with a density of 1.005-1.007 and a refractive index of 1.33. The amount of moisture in a person does not exceed 0.2-0.5 ml. The aqueous humor produced by the processes of the ciliary body contains salts, ascorbic acid, and trace elements. Drainage system
The drainage system is the main route for the outflow of intraocular fluid. Intraocular fluid is produced by processes of the ciliary body. Each process consists of stroma, wide thin-walled capillaries and two layers of epithelium. Epithelial cells are separated from the stroma and from the posterior chamber by outer and inner limiting membranes. The cell surfaces facing the membranes have well-developed membranes with numerous folds and depressions, like secretory cells. Let's consider the ways of outflow of intraocular fluid from the eye (hydrodynamics of the eye). The transition of intraocular fluid from the posterior chamber, where it first enters, into the anterior one, normally does not encounter resistance. Of particular importance is the outflow of moisture through the drainage system of the eye, located in the corner of the anterior chamber (the place where the cornea passes into the sclera, and the iris into the ciliary body) and consists of the trabecular apparatus, Schlemm’s canal, collector canals, intra- and episcleral systems venous vessels. The trabecula has a complex structure and consists of the uveal trabecula, the corneoscleral trabecula and the juxtacanalicular layer. The first two parts consist of 10-15 layers formed by plates of collagen fibers, covered on both sides by the basal membrane and endothelium, which can be considered as a multi-tiered system of slits and holes. The outermost, juxtacanalicular layer is significantly different from the others. It is a thin diaphragm made of epithelial cells and a loose system of collagen fibers impregnated with mucopolysaccharides. That part of the resistance to the outflow of intraocular fluid that falls on the trabecula is located in this layer. Next comes Schlemm's canal or scleral sinus, which was first discovered in the bull's eye in 1778 by Fontan, and in 1830 Schlemm described in detail in humans. Schlemm's canal is a circular fissure located in the limbus area. On the outer wall of Schlemm's canal there are outlet openings of the collector canals (20-35), first described in 1942 by Ascher. On the surface of the sclera they are called water veins, which flow into the intra- and episcleral veins of the eye. The function of the trabecula and Schlemm's canal is to maintain constant intraocular pressure. Impaired outflow of intraocular fluid through the trabecula is one of the main causes of primary glaucoma.
Aqueous humor circulates along the episcleral and intrascleral venous network of the anterior segmented area of the eyeball. It supports metabolic processes and the trabecular apparatus. Under normal circumstances, the human eye contains 300 mm of the component or 4% of the total volume.
The fluid is produced from the blood by special cells that are part of the structure of the ciliary body. The human eye produces 3-9 ml of the component per minute. The outflow of moisture occurs through the episcleral vessels, uveoscleral system and trabecular meshwork. Intraocular pressure is the ratio of the produced component to the withdrawn component.
Structure
The aqueous humor of the eye is almost 100% water. The dense component includes:
- anorganic components (chlorine, sulfate, etc.);
- cations (calcium, sodium, magnesium, etc.);
- insignificant proportion of protein;
- glucose;
- ascorbic acid;
- lactic acid;
- amino acids (tryptophan, lysine, etc.);
- enzymes;
- hyaluronic acid;
- oxygen;
- a small amount of antibodies (formed only in the secondary fluid).
Symptoms
The amount of fluid inside the eye may change due to the development of eye diseases or when exposed to external factors (trauma, surgery).
If the moisture outflow system is disrupted, a decrease in intraocular pressure (hypotension) or an increase (hypertonicity) is observed. In the first case, it is likely to appear, which is accompanied by deterioration or complete loss of vision. With increased pressure inside the eye, the patient complains of headache, blurred vision, and the urge to vomit.
The progression of pathological conditions leads to the development of disturbances in the process of removing fluid from the organ of vision and its tissues.
Types of glaucoma
Glaucoma is a common disease. It mainly affects people over the age of 40, but juvenile glaucoma and even neonatal glaucoma also occur.
Glaucoma can be congenital or acquired . In the case of congenital glaucoma, newborns exhibit increased IOP, and in some cases, an increase in the size of the eyeball. The cause of this form of glaucoma is genetic defects in the structure of the drainage apparatus of the eye. Acquired glaucoma is much more common.
In addition, there are two main forms of glaucoma: open-angle glaucoma and closed-angle glaucoma.
Open-angle glaucoma is the most common form of this disease (about 90% of cases), in which the outflow of intraocular fluid is impaired due to disturbances in the vascular and drainage systems of the eye. It is noteworthy that at first, a patient with open-angle glaucoma may not feel any discomfort or unpleasant sensations.
Angle-closure glaucoma is much less common (about 10% of cases) and usually develops with farsightedness in people over 30 years of age. Angle-closure glaucoma is characterized by acute attacks of closure of the angle of the anterior chamber of the eye. In this form of the disease, intraocular pressure rises quickly, causing severe pain in the eye, headache, and nausea.
Diagnostics
Diagnostic measures for suspected development of pathological conditions in which the intraocular fluid for some reason is in excess, in deficiency, or does not go through the entire circulation process inside the eye, are reduced to the following procedures:
- visual examination and palpation of the eyeball
(the method allows you to determine visible deviations and location of pain); - fundus ophthalmoscopy
- a procedure to assess the condition of the retina, optic nerve head and vascular network of the eye using an ophthalmoscope or fundus lens; - tonometry
is an examination that allows you to determine the level of change in the eyeball when exposed to the eye cornea. With normal intraocular pressure, deformation of the sphere of the organ of vision is not observed; - perimetry
– a method of determining visual fields using computer technology or special equipment; - campimetry
– identification of central scotomas and size indicators of the blind spot in the visual field.
Signs of glaucoma
Glaucoma is characterized by three main symptoms :
- increased intraocular pressure;
- optic nerve atrophy;
- changes in field of view.
Signs of increased IOP are as follows::
- blurred vision, the appearance of a “mesh” before the eyes;
- the presence of “rainbow circles” when looking at a light source (for example, a glowing light bulb);
- a feeling of discomfort in the eye: a feeling of heaviness and tension;
- slight pain in the eye;
- feeling of eye moistening;
- deterioration of twilight vision;
- minor pain in the eye area.
In order to recognize glaucoma in time, it is important to know its symptoms and the patient’s subjective feelings. Different forms of glaucoma have different symptoms.
With open-angle glaucoma, the patient may not be aware of the developing disease for a long time, and there are no clearly defined symptoms. With this form of glaucoma, peripheral vision is first impaired (the field of view is narrowed), and central vision continues to remain normal for some time. As the disease progresses, the patient loses both peripheral and central vision.
An acute attack of angle-closure glaucoma has characteristic symptoms: a significant increase in intraocular pressure (up to 60-80 mm Hg), severe pain in the eye, headache. Often during an attack, nausea, vomiting, and general weakness may occur. Vision in the affected eye decreases sharply. An acute attack of angle-closure glaucoma is often mistaken for a migraine, toothache, acute stomach illness, meningitis, or influenza. In this case, he may be left without the help that is so necessary in the first hours of an attack.
Glaucoma with normal (low) intraocular pressure occurs in patients with myopia, arterial hypotension due to impaired blood supply to the eye, primarily the drainage apparatus, and the optic nerve. With this form of glaucoma, a decrease in visual acuity, a narrowing of the boundaries of the visual field, and the development of optic nerve atrophy occur against the background of normal IOP.
Treatment
For the above-mentioned disorders, as part of the therapeutic course, the patient is prescribed medications that restore intraocular pressure, as well as medications that stimulate blood supply and metabolism in the tissues of the organ.
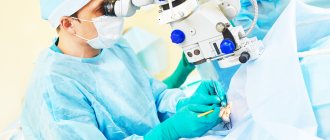
Surgical treatment methods are applicable in cases where drugs do not have the desired effect. The type of surgery performed depends on the type of pathological process.
Thus, the intraocular fluid is a kind of internal environment of the organ of vision. The composition of the element is similar to the structure of blood and provides the functional purpose of moisture. Local pathological processes include disturbances in fluid circulation and deviations in its quantitative indicator.
Aqueous moisture
is a colorless jelly-like liquid that completely fills both.
The composition of aqueous humor is similar to that of blood, only with the lowest protein content. The speed at which a clear liquid is formed is 2-3 µl per minute. During the day, 3–9 ml of liquid is formed in the human eye. Secretion is carried out by ciliary processes, which in their shape resemble long and narrow folds. The processes protrude from the area behind the iris, where the ligaments attach to the eye. The outflow of aqueous humor is carried out through the trabecular meshwork, episcleral vessels and uveoscleral system.
Glaucoma treatment
Conservative treatment of glaucoma includes drugs that reduce the production of intraocular fluid and/or improve its outflow, hemodynamic (improving blood supply) and neuroprotective (protecting nerve fibers) drugs.
These drugs are prescribed only after a diagnostic examination by an ophthalmologist.
If conservative therapy is insufficiently effective (increased IOP, narrowing of the visual field, progression of optic neuropathy), surgical treatment is indicated.
Surgical treatment of glaucoma is aimed at eliminating intraocular blocks (obstacles) in the path of intraocular fluid movement or creating a new outflow path.
There are many types of operations for glaucoma, but the most successful are:
non-penetrating deep sclerectomy
— with drainage of the anterior chamber angle
— without drainage of the anterior chamber angle
After incision of the conjunctiva and formation of superficial and deep scleral flaps, the outer wall of Schlemm's canal is removed, thus increasing the outflow of intraocular fluid through the drainage system of the eye. Sometimes drainage is implanted in the area of excision of the outer wall of Schlemm’s canal to enhance the effectiveness of the operation.
Advantages of this operation:
- painlessness
- local drip anesthesia
- atraumatic
- is carried out without penetration into the eye cavity, which avoids a number of complications (sharp decrease in IOP, bleeding, choroidal detachment, etc.)
Non-penetrating deep sclerectomy is a highly effective method of surgical treatment of open-angle glaucoma.
How does aqueous humor circulate in the eye?
Pathway of aqueous humor outflow
is a complex system in which several structures are involved at once. After aqueous humor is formed by the ciliary processes, it flows into the posterior chamber, and then through into the anterior chamber. Due to the high temperature regime on the front surface, aqueous humor rises to the top and then falls down along the rear surface, which has a low temperature. After this, it is absorbed in the anterior chamber and, through the trabecular meshwork, enters Schlemm’s canal and again into the bloodstream.
Functions of the aqueous humor of the eye
Aqueous moisture
The eye has essential nutrients for the eye, such as amino acids and glucose, which are necessary to nourish the avascular structures of the eye.
Such structures include:
Lens - anterior part - corneal endothelium - trabecular meshwork
The aqueous humor of the eye contains immunoglobulins, through which the protective function of the internal parts of all structures of the eye is carried out.
The constant circulation of these substances neutralizes various factors that can lead to damage to all eye structures. Aqueous moisture
is a medium that refracts light. due to the ratio of formed and excreted aqueous humor.
Causes of glaucoma
The causes of acquired glaucoma can be:
- age-related changes (primary glaucoma);
- eye injury, consequences of inflammation and previous diseases (secondary glaucoma).
Risk factors that increase the likelihood of developing glaucoma include:
- myopia,
- elderly age,
- diabetes,
- thyroid diseases,
- hypotension.
Heredity plays an important role in the occurrence of glaucoma. If your relatives have had glaucoma, you need to be especially vigilant and regularly be examined by an ophthalmologist. An examination by an ophthalmologist and measurement of intraocular pressure at least once a year will allow timely detection and effective treatment of the disease.