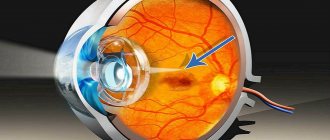
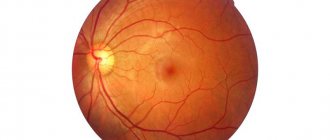
With congenital hypertrophy of the retinal pigment epithelium, we are talking about a violation of the formation of this layer during intrauterine life. The disease manifests itself as grouped pigmentation, which has an external resemblance to the footprint of a bear.
The pathogenesis of retinal hypertrophy has not been fully studied. Some scientists believe that as a result of the formation of macromelanosomes in the pathological retina, a change in catabolic function occurs. As a result, pigment epithelial cells die, and in their place lacunae, or foci of hypogigmentation, form.
Structure
The retinal pigment epithelium is formed by a single layer of hexagonal epithelial cells with a large number of melanosomes containing the pigment melanin[2]. The innermost layer of Bruch's membrane serves as the basement membrane for the pigment epithelium. In the center, near the macula, the epithelial cells are higher; at the periphery of the retina they become somewhat wider and lower. The nuclei of pigmentocytes are located closer to the basal “light” pole; at the apical pole there are a large number of microvilli (cilia) and melanosomes, which seem to wrap the outer segment of photoreceptor cells. There are long and short microvilli. Short microvilli are connected to the ends of the outer segments of the photoreceptors, and long microvilli are located between the outer segments.
The dilator pupillary muscle is derived from the retinal pigment epithelium and its smooth muscle cells are pigmented.
Functions
- Absorption of light. Melanosomes in epithelial cells absorb most of the light that enters the eye and is not absorbed by photoreceptors. The absorption of light rays prevents the reflection and scattering of light across the retina, which helps maintain image contrast and clarity. Under the influence of light, melanosomes of epithelial cells migrate to the apical surface of the cells, into the microvilli, to envelop the outer light-receiving segments of the photoreceptors. In the dark, melanosomes to a certain extent return back to the central part of the cell with the participation of microfilaments and the hormone melanotropin. The function of light absorption is provided mainly by long microvilli.
- Phagocytosis of spent photoreceptor discs. During the activity of photoreceptors, a large number of waste membrane disks with visual pigment are formed. They are subject to phagocytosis by short microvilli of pigment cells. These cells also provide the necessary substances to restore the photoreceptor membrane. Each pigmentocyte phagocytoses 2-4 thousand waste disks every day.
- Storage of vitamin A, a precursor to retinal. When a photon is absorbed, 11-cis-retinal isomerizes into trans-retinal, and an electrical impulse is formed. The restoration of 11-cis-retinal occurs largely with the participation of pigment cells.
- Provides selective supply of necessary nutrients to photoreceptors from the choroid and removal of decay products in the opposite direction. This function is provided primarily by short microvilli, which are combined with the ends of the outer segments of the photoreceptors. The retinal pigment epithelium provides the so-called external blood-retinal barrier, which prevents large molecules from entering the retina from the choriocapillaris.
- Removal of water and ions. Pigment epithelium has the ability to actively remove ions from the intercellular space. Due to the decrease in osmotic pressure, water is also removed. This achieves adhesion of the outer layers of the retina and reduces the possibility of its detachment.
- Removal of excess heat to the choroid.
Thus, the pigment epithelium, photoreceptors and choroid represent a functional unity.
Retina │ Part 1
Description
The retina
has attracted the attention of researchers for many centuries. Chacedon was the first to describe it in 330 BC. e. The name for this structure was given by Rufos Ephesus (ca. 110 AD), who proposed that the retina was a network supporting the vitreous.
For many centuries, not a single researcher thought about the connection between the retina and the brain. It was only Kepler in 1608 who suggested that the retina was the “primary tissue of the visual receptor.”
The first detailed microscopic examination of the retina was carried out by Trevianus in 1835. Subsequent improvements in microscopic techniques, preparation of thin sections and staining methods made it possible to identify the neural organization of the retina, as well as the characteristics of synaptic contacts between neurons and the role of neural connections in the processing of visual information.
The study of the retinal vasculature was facilitated by the development of methods for studying planar preparations of the retina after treatment with trypsin and the use of fluorescein angiography methods. The rapid development of the neuroanatomy of the retina is associated with the development of immunohistochemistry methods
, which make it possible to identify specific substances, in particular neurotransmitters, in a certain structure of the retina with great accuracy. The combination of methods of morphology, immunohistochemistry and neurophysiology (registration of membrane potentials of an individual cell) has now made it possible to obtain a fairly complete picture of the mechanisms of perception and processing of light energy by the retina.
General anatomy
. The retina is part of the inner shell of the eye (tunica internet bulbi) and is a transparent tissue lining the inner surface of the eyeball, occupying 3/4 of its area. It spreads from the optic disc to the dentate line (ora serrata), passing in this area into the pigment epithelium of the ciliary body. The sensory (light-perceiving) part of the retina is adjacent to the retinal pigment epithelium, from which it is easily separated. The strongest connection with the underlying tissues is determined in the area of the dentate line and at the edge of the optic nerve head, near the luteal macula.
In the equator region
the retina has a vertical diameter of 24.08 ± 0.94 mm and a horizontal diameter of 24.06 ± 0.60 mm. The distance from the edge of the optic nerve head to the upper part of the equator is 14.71 ± 1.08 mm, to the lower part - 14.51 ± 1.01 mm. on the nasal side - 13.27 ± 1.11 mm, on the temporal side - 17.29 ± 1.6 mm. Within the indicated boundaries, the area of the mesh shell is 1206 mm2. The anterior part of the retina is examined from the equator to the dentate line. In this case, the distance from the equator to the dentate line on the temporal side is 6.0 ± 1.22 mm, on the nasal side - 5.8 ± 1.12 mm, on the top - 5.07 ± 1.11 mm, on the bottom - 4.79 ± 1.22 mm. The distance from the anterior edge of the retina to the Schwalbe line is 6.14 ± 0.85 mm from above, 6.2 ± 0.76 mm from below, 5.73 ± 0.81 mm from the nasal side, and 6.52 ± 0 from the temporal side. 75 mm.
Microscopic anatomy
. The retina is the most structurally and functionally complex formation of the eye and performs the main function of photoreception. Such a structurally and functionally complex formation can be viewed from different perspectives. For this reason, there are several classifications of its structure - functional classification, histogenetic and anatomical. According to the functional classification, the retina is divided into neurons, glia and the vascular system.
Histogenetic classification differs in that individual retinal structures are subdivided according to the characteristics of their origin. In this regard, derivatives of neuroepithelium (neurons, glia) and mesenchyme (vascular system) are distinguished.
Anatomical classification describes the features of the microscopic structure of the retina. This is what we will focus on in this section. The morpho-functional features of the retina will be given in Chapter 4.
11 layers in the retina.
(Fig. 3.6.1):
Rice. 3.6.1.
Histological structure of the retina:
a - (1 - retina; 2 - retinal pigment epithelium; 3 - section of the choroid); b—layered structure of the retina (high magnification) (1—internal limiting membrane; 2—layer of ganglion cells; 3—inner plexiform layer; 4—inner nuclear layer; 5—outer plexiform layer; 6—outer nuclear layer; 7—outer limiting membrane; 8 - internal segments of photoreceptors: 9 - external segments of photoreceptors; 10 - retinal pigment epithelium)
- Bruch's membrane.
- Retinal pigment epithelium.
- Layer of photoreceptors, rods and cones.
- External limiting membrane.
- Outer nuclear layer.
- Outer plexiform (mesh) layer.
- Inner nuclear layer.
- Inner plexiform (mesh) layer.
- Layer of ganglion cells.
- Layer of nerve fibers.
- Internal limiting membrane.
A number of researchers consider Bruch's membrane simultaneously with the choroid. Histogenetically, Bruch's membrane simultaneously belongs to both the choroid and the retina.
Pigment epithelium
When the inner sensory part of the retina is removed from the inner surface of the eyeball, the pigment epithelium
(pigment part of the retina; pars pigmentosa). It appears as a brown continuous plate extending from the optic nerve to the dentate line. Then it moves to the ciliary body in the form of pigment epithelium. The epithelium in the macula area is the most pigmented. The pigment epithelium of the retina performs diverse functions. It was initially assumed that the pigment epithelium was simply a black background that reduced light scattering during photoreception. At the end of the 19th century. found that separation of the sensory part of the retina from the pigment epithelium leads to loss of vision. This study suggested an important role for the pigment epithelium in photoreception. Numerous recent studies have established the presence of interaction between pigment epithelial cells and photoreceptors. The use of electron microscopy revealed the presence of phagocytic activity of epithelial cells. The use of tissue culture played a certain role in establishing the function of pigment cells.
We will list only some of the functions of the retinal pigment epithelium. More detailed information is given in table. 3.6.1.
Table 3.6.1.
Functions of the pigment epithelium of the retina (according to Linn, Benjamin-Henkind, 1979)
Pigment epithelium contributes to the formation of photoreceptors in embryogenesis
, inducing this process, ensures the functioning of the blood-retinal barrier, maintains the constancy of the environment between the pigment epithelium and photoreceptors, maintains the structure of contact between the outer segments of rods and cones and pigment epithelial cells, ensures active selective transport of metabolites between the retina and the uveal tract, carries out transport, accumulation and isomerization of vitamin A. carries out phagocytosis of the outer segments of photoreceptors, as well as the absorption of light energy by melanin granules, and carries out the synthesis of glycosaminoglycans surrounding the outer segments of photoreceptors.
Pigment epithelial cells phagocytose up to 10% of the outer segments of photoreceptors daily. The ability to phagocytose the outer segments of rods and cones is direct evidence of the constant regeneration of the latter.
The absorption of light energy by melanin granules provides a clear topographic registration of light energy by the outer segments of photoreceptor cells, enveloped in processes of pigment epithelial cells containing melanin grains. This ensures light isolation of each photoreceptor. When the illumination of the eyeball increases, melanin grains migrate into the processes of pigment epithelial cells. At the same time, the degree of isolation of photoreceptors increases.
Absorption and transport of retinol
(vitamin A) is provided by receptors located on the basal and lateral surfaces of pigment epithelial cells. Pigment epithelial cells synthesize a special glycoprotein that transfers retinol into the interphotoreceptor matrix, from where it is received by photoreceptors.
It should be noted that dysfunction of the pigment epithelium underlies the development of a number of diseases. Its structural changes have been identified in age-related maculopathy, central serous retinopathy, and retinal dystrophy. These changes are clearly visible ophthalmoscopically.
Pigment epithelial cells are sensitive to a number of toxins.
The retinal pigment epithelium is located between the choriocapillary layer of the choroid and the sensory part of the retina
(Fig. 3.6.1—3.6.4).
Rice. 3.6.2.
Pigment epithelium of the retina:
a - cross section (1 - outer segments of rods and cones; 2 - cells of the pigment epithelium; 3 - basal lamina (Bruch's membrane); 4 - choroid proper); b—planar preparation
Rice. 3.6.3.
Planar preparation of retinal pigment epithelium. Epithelial cells are polygonal in shape and intensely pigmented
Rice. 3.6.4.
Scanning electron microscopy of the retina (St) and its connections with the pigment epithelium (Pm) (according to Kessel, Kardon, 1979): the
outer segments (He) of the photoreceptors are in contact with individual cells of the pigment epithelium (I, II).
Vacuoles (Vc) in pigment epithelial cells appear as a result of the loss of melanin grains during histological processing of tissues. The lower left shows a higher magnification of the area shown in the box in the top image. Bottom right shows the basal surface of pigment epithelial cells after removal of Bruch's membrane. A junctional complex in the form of bridges is visible between the cells. It represents one layer of flattened, intensely pigmented cells, tightly adjacent to each other and having a hexagonal shape (Fig. 3.6.2; 3.6.3). Cell sizes vary widely depending on their location. In the foveal region they are higher (height 14-16 µm), narrower (10-14 µm) than in the area of the dentate line (width 60 µm).
Cells lying along the periphery are flattened and less pigmented. Near the dentate line there are multinucleated cells, and there are fewer melanin grains.
At the time of birth
In humans, about 4-6 million cells are found. As the body develops, the density of pigment epithelial cells increases in the macula area, reaching a maximum at 6 months. Conversely, in the area of the dentate line, the number of cells decreases rapidly during the first year of life.
With age, pigment cells in the macula area increase in height and decrease in width. The opposite pattern is found along the periphery of the retina. Figures of mitotic divisions in the epithelial layer are practically not detected.
Cell structure
.
As in any epithelial cells of the human body, the cells of the pigment epithelium of the retina are distinguished between apical and basal parts. On the basal side they are adjacent to the basement membrane
(Fig. 3.6.5).
Rice. 3.6.5.
Features of the ultrastructural organization of retinal pigment epithelial cells and contacts between cells:
1 - cytoplasmic processes;
2 - functional complex located between adjacent cells: 3 - Bruch's membrane: 4 - connective tissue Under light microscopy, the tissue lying between the pigment epithelium and the choriocapillaris layer of the choroid of a homogeneous structure was called the vitreous plate
(lamina vitrea), later it was called Bruch's membrane (compexus (lamina) basalis (Bruch)).
Using more precise methods of light microscopy, the following parts were identified in Bruch's membrane: the outer cuticular part and the more fibrous inner part. Because the interior of Bruch's membrane stains intensely when using methods that reveal elastic tissue, it is called "lamina elastica". The structural features of Bruch's membrane
and its thickness depend both on the location of the area under study and on the age of the individual. In adults, the thickness of the membrane in the peripapillary region is 2-4 µm, and in the peripheral region - 1-2 µm. In children, its thickness in the central areas is 2 microns.
Ultrastructural studies have made it possible to identify five layers (zones)
:
- basement membrane of the pigment epithelium,
- inner collagen layer,
- layer of fibers (elastic),
- outer collagen layer
- basement membrane of choriocapillaris endothelial cells (Fig. 3.6.6 - 3.6.8).
Rice. 3.6.6.
Volumetric schematic representation of the inner layer of the choroid and the retinal pigment epithelium, between which Bruch’s membrane is located (according to Hogan et al., 1971):
1 - cytoplasmic processes of pigment epithelium cells; 2 - outer segment of the stick; 3 — locking tape; 4—desmosome; 5 - nucleus of a pigment epithelial cell; 6 - mitochondria; G - Golgi complex; 5 - pigment granules; 9 - phagosomes; 10—smooth endoplasmic reticulum; 11—basal membrane; 12—elastic zone of Bruch's membrane; 13 collagen fibrils of Bruch's membrane; 14 — choriocapillaries of the choroid (the arrow indicates the pores); 15 - collagen fibers located between the capillaries of the choroidRice. 3.6.7.
Scheme of the structural organization of Bruch's membrane (according to Hogan et al., 1971):
1 - basement membrane of the retinal pigment epithelium; 2—anterior collagen zone; 3 - elastic layer; 4 - outer collagen layer: 5 - basement membrane of the choriocapillaris: b - pigment epithelium; 7 - endothelial cell of the choriocapillarisRice. 3.6.8.
Ultrastructure of Bruch's membrane:
1 - basement membrane of pigment epithelial cells; 2 — internal collagen layer of Bruch’s membrane with a thickness of 2.5 μm; 3 - elastic layer of Bruch's membrane; 4 - outer collagen layer 0.7 microns thick. 5 - basement membrane of endothelial cells of the choriocapillary layer of the choroid; 6 - endothelial cell
In fact, Bruch's membrane can be considered to consist of only three inner layers, since the outer layers belong to other formations.
The innermost layer of the membrane
, represented by the basement membrane of the retinal pigment epithelium, has a thickness of approximately 0.3 μm. The inner collagen zone (1.5 µm thick) consists of densely packed and strictly oriented collagen fibrils (fiber diameter 60 nm, striation periodicity 64 nm). Collagen refers mainly to type IV collagen. The fibers are immersed in a ground substance consisting primarily of proteoglycans.
Middle zone
(elastic layer) has a thickness7 of the order of 0.8 microns, and in it the elastic fibers are arranged randomly. It is in this zone that during aging and various pathological conditions the accumulation of calcium salts and lipids is observed.
Outer collagen zone
similar in structure to the inner zone. The only difference is that it is thicker (0.7 microns).
Outermost layer of Bruch's membrane
, represented by the basement membrane of the endothelial cells of the capillaries of the choroid. the thinnest (0.14 microns).
Drusen can often be detected in the area of Bruch's membrane and pigment epithelial cells during ophthalmoscopy.
, developing as a result of aging processes or various diseases (Fig. 3.6.9).
Rice. 3.6.9.
Formation of drusen in the inner collagen layer of Bruch's membrane:
1 - pigment epithelial cells;
2—part of the drusen located in the inner collagen layer; 3 - the outer part of the drusen, spreading over a long distance (arrows) There are hard and soft drusen
. They may appear or regress. Hard drusen are more common in young people and are a product of the synthetic activity of pigment epithelial cells. Soft drusen, containing membrane structures, reflect general dysfunction of cells.
Bruch's membrane performs diverse and important functions, primarily the selective transport of nutrients and water towards the retina. It is Bruch’s membrane, together with the choriocapillary layer of the choroid and pigment epithelial cells, that forms a unique structural and functional unit
, providing barrier functions. Violation of the structure of the membrane is the cause of various degenerative diseases of the pigment epithelium (epithelial detachment) and the sensory part of the retina (tapetoretinal degeneration, degeneration of the macular region, etc.). This is facilitated by age-related changes and the formation of drusen.
Continuing the description of pigment epithelial cells, it is necessary to point out that they, like other epithelial cells, form numerous folds in their basal part. On the apical surface of the cells, many microvilli are detected, extending in the space between the outer segments of the photoreceptors and enveloping them. There are two types of microvilli
. The first type has a length of 5-7 microns, and the second - 3 microns. Microvilli significantly increase the area of contact of pigment epithelial cells with photoreceptors, thereby promoting a high level of metabolism by increasing the intensity of the supply of nutrients to the retina from the choriocapillaris layer of the choroid and the removal of water, ions and metabolic end products from the retina.
There are no specialized connections between the cytoplasmic membrane of microvilli of epithelial cells and the membrane of photoreceptors and a slit-like space
(Fig. 3.6.10).
Rice. 3.6.10.
Electron diffraction diagram illustrating the nature of the relationship between the retinal pigment epithelium and the outer segments of the rods (according to Hogan et al., 1971):
1 - nucleus of the pigment epithelium cell;
2 - mitochondria; 3 - smooth endoplasmic reticulum; 4 - melanin granules; 5 - microvilli located on the apical surface of the pigment epithelium cells and surrounding the outer segments of the rods; 6 - outer segment of the photoreceptor. This space is filled with a “cementing substance” of a complex chemical composition. They call it the “interphotoreceptor matrix.” It is synthesized by pigment epithelial cells. The interphotoreceptor matrix consists of chondroitin sulfate (60%), sialic acid (25%) and hyaluronic acid (15%). The composition and functions of this substance have now been clarified.
It was initially assumed that the matrix
is a homogeneous accumulation of proteoglycans. Currently, a rather complex spatial interaction of matrix proteoglycans with the outer segments of cones has been revealed. It is this interaction that ensures fairly tight contact between the pigment epithelium and the retina. The interphotoreceptor matrix is involved in retinal metabolism, namely retinoid transport. It also promotes phagocytosis of the outer segments of photoreceptors.
Violation of the structural organization of the matrix is an important cause of retinal detachment, and also accompanies various types of retinal degeneration.
Pigment epithelial cells are tightly connected to each other using closure zones, encircling desmosomes and gap junctions. Organelles surround the cells on the apical side, tightly holding them together. Desmosomes are located in the middle part of the cells. Such contact makes it impossible for metabolites, especially high-molecular substances, to pass along the intercellular space. This transfer occurs only through the cytoplasm of the cell in an active way. It is this tight intercellular contact that allows the blood-retinal barrier to function.
In different parts of the pigment epithelial cell, the cytoplasm has a different ultrastructural structure
. It is for this reason that the cytoplasm of the cell is conventionally divided into 3 zones. In all zones, a well-developed agranular endoplasmic reticulum is determined.
The outer third of the cytoplasm of epithelial cells is characterized by the presence of a large number of mitochondria and folds of the basement membrane
. The inner third of the cytoplasm of epithelial cells is saturated with melanin granules. Numerous free and bound ribosomes are also visible. The intermediate zone of the cytoplasm is relatively poor in organelles (Fig. 3.6.10). This is where the core is located. The Golgi complex is not clearly expressed. Its cisterns contain light material, which indicates high secretory activity of the cells.
Lysosomes of normal structure are located in all parts of the cytoplasm of epithelial cells.
. Their main function is the enzymatic breakdown of phagocytosed fragments of the outer segments of photoreceptors.
Since the phagocytic activity of pigment epithelial cells is one of the main functions, their cytoplasm contains phagolysosomes, formed as a result of the fusion of the absorbed outer segments of photoreceptors with the primary lysosome.
In the phagolysosome, the protein component of the photoreceptor disks is the first to undergo lysis.
Process of phagocytosis and lysis
segments of the external segments of the photoroceptors occurs quite quickly. One rabbit pigment epithelial cell per day lyses 2000 discs in the parafoveolar region of the retina, 3500 discs in the perifoveolar region and almost 4000 along the periphery of the retina (Fig. 3.6.11, 3.6.12).
Rice. 3.6.11.
Electron diffraction diagram illustrating the stages of digestion of fragments of the outer segments of photoreceptors by pigment epithelial cells:
1 - outer segment of the cone; 2 - separated fragment of the outer segment of the cone and immersed in the cytoplasm of the pigment epithelial cell; 3 - phagosome. containing a fragment of the external segment of the cone: 4 - phagosome at a later stage of digestion of the fragment of the external segment: 5 - melanosomes; 6 - mitochondria
Rice. 3.6.12.
Consecutive stages (I-VI) of absorption and lysis of the outer segments of photoreceptors by pigment epithelial cells of the retina.
In this case, regeneration of the outer segment of the photoreceptor is noted: 1 - outer segment of the photoreceptor;
2 - pigment epithelial cell; 3 - phagosome It was noted that with intense lighting the number of phagosomes increases. Pigment epithelial cells shed the outer segments of cones in the same way as rods, but more intensely after the cessation of illumination. The process of destruction of the outer segments of cones and rods of photoreceptors and their utilization is an adaptive mechanism that helps maintain the structural and functional integrity of the photoreceptor apparatus. However, the death of photoreceptors also occurs in various pathological conditions. Cell death often occurs due to apoptosis mechanisms under genetic control.
Recently, intensive research has been carried out on the role of apoptotic mechanisms in the development of a large group of inherited degenerative diseases of the retina. This area of research is of great practical importance, since more than 100 genetically inherited syndromes are known that are accompanied by the death of retinal neurons. It has been shown that in some inherited syndromes, apoptosis mechanisms play a leading role. In this case, apoptosis is considered as the final mechanism of cell death
, regardless of the nature of the primary damage. The main types of damage to photoreceptors are quite diverse and boil down to disruption of their important functions (synthesis of visual pigment, the structure of the cytoskeleton of cells, the sequence of processes during the perception of light energy and its transformation into a nerve impulse, phagocytic functions of pigment epithelial cells, etc.). Discovering the mechanisms of apoptotic death of retinal neurons and the participation of the genetic apparatus in this is considered as the most promising direction in the treatment of these diseases.
A common structural inclusion in the cytoplasm of the retinal pigment epithelial cell is lipofuscin.
Lipofuscin is found in many tissues of the body and its amount increases with age. It is for this reason that this pigment has been called the “aging pigment.”
. It occurs as a result of the accumulation of non-lysing protein and lipid aggregates in the lysosomes of aging cells. This pigment has characteristic physicochemical properties, including a natural yellowish-green fluorescence. The accumulation of lipofuscin occurs not only during the aging process, but also in a number of metabolic diseases. The causes and mechanisms of lipofuscinosis have remained a mystery for more than 100 years. It is now known that lipofuscin arises from the peroxidation of cellular components, especially lipids.
In the eyeball, as mentioned above, lipofuscin is found in the retinal pigment epithelium
. Its maximum accumulation occurs in cells located in the posterior pole. By the age of 80, lipofuscin granules occupy up to 19% of the volume of epithelial cells. Unlike other cells of the body, in which lipofuscin arises as a result of autophagocytosis of intracellular organelles, lipofuscin in retinal pigment epithelial cells arises as a result of phagocytosis of the outer segments of photoreceptors, followed by peroxidation of the lipid fraction of these fragments. This process involves the short-wave spectrum of light energy.
Recently, vitamin A and its derivatives have been shown to play a major role in the formation of lipofuscin in retinal epithelial cells. This is evidenced by numerous experimental biochemical and physicochemical studies.
Lipofuscin grains must be morphologically distinguished from melanosomes
. This is of practical importance in the diagnosis of pigmented neoplasms. Melanin granules of epithelial cells are round or oval in shape. In this case, round granules are located in the apical part of the cell, and oval granules are located in the microvilli. Lipofuscin granules are round but less electron dense. They are stained with Sudan dyes and fluoresce. The number of lipofuscin grains progressively increases with age. On the contrary, the number of melanosomes decreases with age. It is believed that the decrease in the number of melanosomes is associated with the activity of the lysosomal apparatus of cells and age-related changes in melanin.
Melanin in pigment epithelial cells absorbs light energy of a fairly wide spectrum, protecting photoreceptors and the cytoplasm of pigment epithelial cells from the damaging effects of light. Melanin has the properties of a free radical and functions in the same way as a polymer involved in the exchange of electrons. Melanin binds a number of metals and medicinal substances.
It is also important to remember that the melanin granules of the retinal pigment epithelium differ from the melanosomes of the stromal melanocytes of the uveal tract
. Uveal melanin granules are much smaller and oval in shape. This is important for pathologists to know, especially in the differential diagnosis of intraocular pigmented neoplasms.
In the apical part, as well as near the Golgi complex of pigment epithelial cells, a large number of pinosomes
. Their size is smaller (53 nm) than in endothelial and other cells (more than 100 nm). These structures indicate the presence of intensive processes of endocytosis, characteristic of pigment epithelial cells.
Discrete dark particles and lamellar bodies can also be found in the cytoplasm of epithelial cells
. The latter are fragments of absorbed outer segments of photoreceptors.
Continued in the next article: Retina? Part 2
—-
Article from the book: Structure of the human visual system | Vit V.V.
Clinical significance
In albinos, melanin synthesis is impaired, and there is almost no melanin in the pigment layer. When albinos are in a brightly lit room, the light entering the eyeball is reflected in all directions by the non-pigmented surface of the retina and underlying tissues. This results in the stimulation of a large number of rods and cones by one single beam of light, although in a healthy person only a few photoreceptors are excited. Visual acuity in albinos, even with the best optical correction, rarely exceeds 0.2-0.1 (norm 1.0)[4].
During life, the pigment epithelium accumulates end products that are not completely decomposed - lipofuscin; it is also deposited between the pigment epithelium and Bruch's membrane in the form of drusen. Drusen are a sign of the development of age-related macular degeneration.
Disorders of the retinal pigment epithelium also occur in retinitis pigmentosa.
When the blood-retinal barrier is disrupted (for example, in diabetes mellitus), diabetic retinopathy develops.
Diagnosis of the disease
It is quite difficult to diagnose this disease in young children and in the early stages of development. In children, a diagnosis can only be made after the child reaches the age of 6 years. Most often, the disease is detected when the child begins to experience certain difficulties at night or at dusk.
To confirm or refute the diagnosis of retinal dystrophy, it is necessary to undergo a thorough examination of the visual system. Diagnostics are performed using modern computer technology, which allows us to create a complete picture of the patient’s vision.
If retinal dystrophy is suspected, the patient's examination should include a number of measures.
- Determination of visual acuity.
- Perimetry (visual field examination) to assess the condition of the retina at its periphery - you can determine which area of the retina is affected.
- Tonometry (measurement of pressure inside the eye).
- Ophthalmoscopy (examination of the fundus of the eye).
- Electrophysiological study - determines the viability of retinal neurons and the optic nerve.
- Fluorescein angiography allows you to visualize blood vessels and assess their condition.
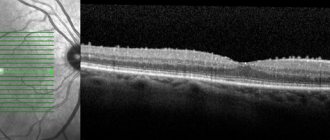
- OCT (optical coherence tomography) is a non-invasive method for studying the thin layers of human eye tissue, in which a layer-by-layer examination of the structures of the eye is performed.
- Ultrasound (ultrasound A- and B-scanning) of the internal structures of the eye.
Notes
- ↑ 12
Foundational Model of Anatomy - ↑ 1 2 Cassin, B. and Solomon, S.
Dictionary of eye terminology (undefined). - Gainesville, Fla: Triad Pub. Co, 2001. - ISBN 0-937404-63-2. - Boyer MM, Poulsen GL, Nork TM. "Relative contributions of the neurosensory retina and retinal pigment epithelium to macular hypofluorescence." Arch Ophthalmol. 2000 Jan;118(1):27-31. PMID 10636410.
- Guyton A.K., Hall D.E.
Medical physiology = Textbook of Medical Physiology / ed. IN AND. Kobrina. - M.: Logosphere, 2008. - P. 699. - 1296 p. — ISBN 978-5-98657-013-6.