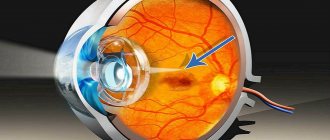
Epiretinal membrane is a retinal disease in which a film forms on the retina of the eye that has contractile properties. The epiretinal membrane is not formed over the entire retina, but only in its central part, the macula. With ophthalmoscopy of the fundus, the epiretinal membrane looks like a thin, whitish, transparent and wrinkled formation, very similar to cellophane film.
In fact, this is what initial scar changes look like, which, as they progress, tighten the retina, causing its deformation and the formation of rough folds. The initial stages of epiretinal membrane do not lead to damage to the outer layers of the retina and photoreceptors, so the disease is often asymptomatic and does not affect visual acuity in any way.
As the film on the retina thickens (ripes) and becomes rougher, various complications begin to appear, for example, swelling and rupture of the retina, which have a pronounced clinical picture and become the reason for visiting a doctor. The disease is characterized by a long course and slow progression. In the vast majority of cases, the epiretinal membrane of the eye is detected in patients 55-65 years of age.
Causes of development of the epiretinal membrane of the eye
The appearance of an epiretinal membrane sometimes cannot be associated with any clear causes. In this case, the disease is said to be idiopathic. However, there are a number of ophthalmological diseases and conditions, the presence of which is accompanied by the development of the epiretinal membrane of the retina. These include:
- Traumatic eye injuries.
- Age-related involution of the vitreous body.
- Hemorrhage into the vitreous body.
- Diabetes mellitus complicated by proliferative retinopathy.
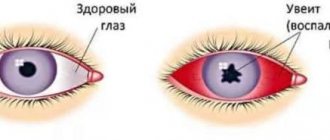
- Inflammation of the choroid of the eye (uveitis).
- Thrombosis of retinal vessels, in particular the central vein.
- Epiretinal macular membrane can be a consequence of treatment of various ophthalmological diseases, especially if surgical techniques were used.
Symptoms and diagnosis of retinal film
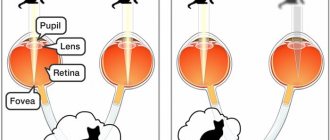
As noted earlier, the epiretinal membrane of the eye is formed only in the macular region of the retina, which is responsible for central vision. Accordingly, the main clinical manifestations will relate specifically to central vision impairment. Typically, patients complain of decreased image sharpness, a feeling of fog in the eyes, difficulty reading, distortion of the contours of objects, and double vision.
The degree of manifestation of these symptoms can vary from slight to very strong, depending on the density of the membrane and area, the severity of its traction effect on the underlying retina. Most often, changes are observed only in one eye, but bilateral damage is also possible.
| Amsler test normal | Self-control using the Amsler grid | Curvature of straight lines |
In order to visualize the epiretinal membrane and make a diagnosis, standard ophthalmoscopy, which involves examining the fundus, is sufficient.
There are also specialized diagnostic techniques that allow you to obtain comprehensive information about the state of the eye structures or detect the membrane in unusual situations. For example, if a patient has clouding of the optical media of the eye, then ophthalmoscopy will not allow clear visualization of the fundus. In this case, it is possible to identify the epiretinal membrane through ultrasound examination of the eye.
| Epiretinal membrane of the eye | Characteristic ophthalmoscopic picture |
In rare cases, when there are symptoms of macular edema, fluorescein angiography may be prescribed, with the help of which this pathology is verified.
But one of the most advanced and informative examination methods is coherence tomography. It can be used to diagnose epiretinal membrane in the early stages when other methods do not allow this.
| OCT diagnostics of dense epiretinal membrane |
Patients also undergo a standard ophthalmological examination, including determination of visual acuity, the Amsler test and other ophthalmological tests that reveal the degree of central vision impairment.
Diagnostics
The epiretinal membrane can be detected during fundus examination (ophthalmoscopy). The formation has the appearance of a cobweb-like film, often resembling cellophane, as a result of which this disease is sometimes called “cellophane retinopathy.”
In patients in whom ophthalmoscopy is difficult (cataracts, severe destruction of the vitreous, corneal opacities), ultrasound methods of eye examination and optical coherence tomography will help identify the epiretinal membrane and clarify its size and structural features.
Signs of macular damage can be detected using the Amsler grid (curved lines) and visual acuity testing.
Definition, classification, prevalence of idiopathic epiretinal membrane
Epiretinal membrane (ERM) is one of several conditions of the vitreomacular interface that characterizes the pathological interaction of the retina and vitreous structures (VT). The pathology of the vitreomacular interface also includes posterior vitreous detachment (PVD), vitreomacular traction syndrome, lamellar macular hole, macular pseudo-hole, pseudo-operculum, macular hole, macular micro-hole [1, 2], etc., however, according to the authors, ERM is involved in the pathogenesis of each of these conditions of the vitreoretinal interface [2].
The ERM is histologically a collection of cells and extracellular substances that form a membranous structure on the vitreal surface of the retina [3]. Having direct contact with the retina and ST and participating in the dynamic process of vitreoretinal interaction, ERM often causes a significant decrease in central vision, as well as the occurrence of symptoms such as metamorphopsia, macropsia, micropsia, aniseikonia and even monocular diplopia.
The first description of the fibrocellular membrane structure on the surface of the internal limiting membrane (ILM) was published by A. Iwanoff in 1865 [4].
However, at present there are many synonyms both in domestic and foreign literature that define the same condition (traction maculopathy, macular pucker, macular pucker syndrome, cellophane macular reflex, cellophane maculopathy (CM), preretinal macular fibrosis, macular fibroplastic syndrome, central edematous fibroplastic syndrome, superficial fold retinopathy) [5-7], which reflects numerous aspects of the clinical picture and/or pathogenesis of this condition. The most universal term for describing the membrane structure on the surface of the retina has become “epiretinal membrane”.
Depending on the presence or absence of eyeball diseases associated with ERM, the following forms are distinguished:
— idiopathic (primary) epiretinal membrane (iERM), first described by H. Kleinert in 1955;
- secondary ERM (sERM), associated with the following conditions: vascular diseases of the retina, inflammatory diseases of the eyeball, condition after injury to the eyeball, previous surgical interventions (cataract surgery and retinal detachment), condition after a large volume of laser coagulation of the retina as part of proliferative diabetic retinopathy ( PDR), proliferative vitreoretinopathy (PVR) [2, 8].
Based on the ophthalmoscopic picture, J. Gass proposed a classification of ERM [9], slightly modified by R. Klein et al. [8] taking into account color photographs of the fundus.
Based on the relationship between the identified changes in the macular zone and visual functions, ERM is currently classified into CM or cellophane macular reflex and preretinal (epiretinal) macular fibrosis (PMF) or macular folding. CM, as an early manifestation of ERM, is usually asymptomatic. More severe manifestations of ERM, referred to as PMF, can significantly reduce visual function [10, 11].
The prevalence of ERM has been studied in large epidemiological studies using examples from Australian, Japanese, Chinese and other populations [7, 8, 12, 13]. According to these studies, the highest incidence of ERM was recorded in the Hispanic population of the United States (18.5%), and the lowest in China (2.2%) [13]. The incidence of iERM in population studies was 5.4% of all ERM [2, 14]. Some authors [7] note a significant increase in the incidence of ERM with age; it reaches 2.6% in the population aged 50-59 years, 9.4% in those aged 60-69 years, and about 15% and even 20% in those aged 70-79 years. . The relationship between age and the incidence of ERM, according to the authors [8], is explained by an increase in the frequency of events such as PVD, cataract surgery and other retinal diseases, which increases the risk of developing ERM. In addition, a more pronounced form of manifestation of ERM, PMF, was more often registered in 65-74 years.
No significant differences in frequency of occurrence were found in male and female populations [7, 8, 10]. According to the Handan Eye Study, PMF occurs significantly more often in women than in men [10].
The incidence of ERM in one eye in the population according to the Beaver Dam Eye Study was 11.8%, in both eyes - 2.4% [8].
The incidence of CM was 4.8%, and PMF was 1.7% [7]. A decrease in visual acuity significantly correlated with the detection of PMF and CM in the population [7].
The relationship between the occurrence of vERM and certain diseases and operations on the eyeball has been proven [2, 8]. No relationship has been identified between ERM and the presence of posterior capsular cataracts [8], glaucoma [7, 10], or narrowing of arterioles in the fundus [8]. The relationship between the frequency of occurrence of ERM and different types of ametropia was also studied.
In the Chinese population, a significant correlation was found between myopia and the frequency of iERM, which the authors explain by the higher frequency of PVD, one of the possible pathogenetic mechanisms of the formation of ERM, in the population with myopia [10], while according to the Beaver Dam Eye Study, no correlation with ametropia was identified [8] . The Beaver Dam Eye Study found that the incidence of another vitreomacular pathology, macular hole, was higher in eyes with installed ERM [8]. In addition, neither the presence nor severity of ERM manifestations, according to the Beaver Dam Eye Study and Handan Eye Study, was associated with such systemic factors as arterial hypertension and other cardiovascular diseases, smoking or alcohol consumption [8, 10]. There was also no correlation with body mass index, ankle-brachial systolic pressure index, finger-brachial pulse wave velocity index, fasting blood glucose, serum cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, urea, creatinine, and even educational level [10 ].
Modern ideas about the pathogenesis of the epiretinal membrane
The pathogenesis of ERM development was first proposed in 1971 by A. Roth and R. Foos [15], who expressed the opinion about the migration of retinal glial cells through defects in the ILM and their proliferation on the surface of the retina with the formation of a fibrocellular membrane. More recent studies also suggest that ERM is a result of fibroglial proliferation secondary to ILM ruptures due to PVD [1].
Currently, two hypotheses for the formation of ERM are justified.
1. In their hypothesis of the pathogenesis of ERM development, A. Roth and R. Foos [15] expressed the opinion of the migration of retinal glial cells through defects in the ILM and their proliferation on the surface of the retina with the formation of a fibrocellular membrane.
2. According to another hypothesis, hyalocytes from the CT cavity migrate to the surface of the retina and synthesize collagen, which leads to the formation of a fibrous membrane [6, 16]. The fibrocyte-like and macrophage properties of these cells, as well as the detection of type II collagen on the surface of the ERM, allow us to conclude that hyalocytes are involved in its formation [6].
Nevertheless, a large amount of data indicates that the formation of the ERM is not only associated with biomechanical interaction at the CT-retina interface and the ability of most cells to migrate and proliferate, but that it is a complex process occurring at the macro- and microlevel with the involvement of many molecular cascades reactions and changes in microcirculation.
Along with characterizing the histological composition of the ERM, for a more accurate understanding of the formation of iERM, it is worth highlighting the interaction of the following pathogenetic links: PVD, ILM, microcirculatory disorders, molecular mechanisms.
Histological characteristics of ERM
Under light microscopy, the ERM appears as a membrane structure 4-5 µm thick, with a weak eosinophilic color, with linearly arranged mononuclear cells with an oval nucleus and unclearly visualized cytoplasm [2]. Histological studies of ERM removed during vitrectomy indicate various combinations of cells in its composition: retinal glial cells, Müller cells (CM); fibrous astrocytes; fibrocytes; myofibrocytes; retinal pigment epithelial cells (RPE); hyalocytes; inflammatory cells and macrophages [9, 16, 17]. All of these cells have been shown to undergo metaplasia, which often complicates their identification [2]. Below is a brief description of cells related to the formation of the ERM.
Müller cells (CM) are radially oriented macroglial cells extending from the external limiting membrane (ELM) to the vitreal surface of the retina. CMs perform a number of important local functions: they stabilize the architectonics of the retina, participating in the formation of the NLM and ILM, ensure the metabolism of retinal neurons, and prevent the migration of photoreceptors in the subretinal space. In vivo
and
in vitro,
CMs can produce and/or express various cytokines, growth factors and receptors, cell cytoskeletal proteins—intermediate filaments.
Under in vitro
conditions, CMs express messenger RNA encoding types I-VII, IX and XI of collagen [18], including types II, V, XI, VI and IX - collagen CT, types IV and VI - collagen in the ILM, VII type - collagen, described in ERM [19]. Immunohistochemical studies support the idea of the glial origin of cells in the iERM [6]. The result of analysis of all ERMs for glial fibrillary acidic protein (GFAP), a component of intermediate microfilaments of the cell cytoskeleton, as well as glutamine synthetase (GS), an enzyme specifically expressed in BM, is positive [6].
According to electron microscopy data in a study by M. Patronas et al. [2], most of the identified cells in the ERM are fibrous astrocytes. Apparently, this is partly explained by the fact that in the central zone of the retina, the location of the ERM, where the retinal thickness is more than 130 µm, non-radial glial cells, most of them astrocytes, appear in the layer of nerve fibers [20]. These are large spindle-shaped cells that tend to proliferate in a monolayer. Fibrous astrocytes can undergo myoblastic differentiation, which is confirmed by the presence of local accumulations of filaments (5-7 nm) and spindle-shaped densities along the edge of the cytoplasm [5]. The most common opinion is that cells of glial origin dominate in the iERM, although some authors note the presence of both glial and RPE cells as the main cells [5]. The significant role of other cell types in the formation of the ERM is also determined by the fact that the ERM, consisting only of glial cells, can remain asymptomatic until additional types of cells appear in the membrane: RPE cells, fibrocytes, myofibrocytes and macrophages [16], which probably provide it the ability to reduce and, consequently, the appearance of clinical symptoms of ERM.
Each of the listed cell types (fibrous astrocytes, fibrocytes, myofibrocytes, RPE cells, macrophages) included in the formation of the ERM has myofibroblastic potential, which may explain the contractility of this membrane [9, 16].
Not only the qualitative cellular composition, but also various quantitative combinations of cells in different types of ERM can probably determine its proliferative potential and ability to contract, which is clinically manifested by the progression and severity of disease symptoms. Using immunohistochemical methods and specific markers, a number of authors have studied the proliferation of cells in various types of ERM [17] removed during surgical treatment of the disease in the phase of its active clinical manifestations. Thus, the duration of the disease from the moment of detection of its first signs to the moment of surgical treatment turned out to be the shortest in cases of PVR (on average 1.5 months), and the longest (on average 11.2 months) in cases of iERM [17].
In order to quantify the proliferative potential of the ERM, the authors used the term “proliferative index”—the ratio of the total number of cells to the number of dividing cells [21]. To identify dividing cells and their origin, all membranes were labeled with antibodies to the Ki-67 protein (a nuclear protein expressed in proliferating cells), to identify proliferating cells - with specific antibodies to glial (marker - intermediate filament proteins: GFKB and vimentin), immune (marker for microglial cells and macrophages - plant hemagglutinin Ricinus communis
) cells and RPE cells (marker - ezrin, a protein of the cytoskeleton of RPE microvilli) [17]. It was revealed that the clinically rapidly growing ERM (within the framework of PVR) has the highest cell density and the largest number of anti-Ki-67 labeled cells. In this study, membranes termed “old” (i.e., iERM) had much lower levels of Ki-67 identified cells, indicating a less reactive nature of proliferation in the iERM at the time of its active clinical manifestation. According to the results of an immunohistochemical study, a significant accumulation of proliferating RPE cells was detected in the membranes of PVR and significantly fewer RPE cells in the membranes of PDR and iERM. Interestingly, in iERM and PDR there was no history of retinal tears or surgical history, but proliferating RPE cells were present in these membranes, which provides strong support for the hypothesis that RPE cells can migrate through the intact retina. Proliferating glial cells were found in all types of ERM. The number of glial cells in the proliferation phase per unit area (1 mm2) in cases of iERM prevailed over the number of other cells in the proliferation phase per 1 mm2 [17], which confirms the main opinion about the predominance of glial cells in the formation of iERM.
There is also a significant inflammatory component in all ERMs, as detected by ricin labeling. The presence of macrophages, lymphocytes and monocytes in the ERM has been noted previously, but no study has been carried out on the number of cells involved in the response. It is unknown whether the immune activation in this case is a response to the initial microtrauma due to diseases or PVD, or whether it is an autoimmune reaction, possibly caused by a violation of the blood-retinal barrier. In addition, the immune cells found in all types of membranes also turned out to be actively proliferating. The data obtained are consistent with the hypothesis about the parallel course of two processes: healing and formation of the ERM [17]. ERM formation likely involves two phases: an early proliferative phase with many proliferating cells and a slow proliferating phase with a less reactive membrane. After the proliferation phase, a contraction phase occurs with deposition and contraction of the extracellular matrix [22]. This hypothesis is confirmed by the fact of the low proliferative potential of iERM at the time of its active clinical manifestations, coinciding with the contraction phase.
The role of PVD
PVD is a gradual process, the development of which clearly correlates with age-related changes in TS. Data from histological studies indicate an important role of the PVD process in the pathogenesis of iERM [9]. It was later found that approximately 75% of patients with ERM have a complete PVD [9]. According to some authors, the development of ERM and other pathology of the vitreomacular interface is associated only with partial posterior vitreous detachment (PVD) [14]. In a study by S. Chung et al. [23] describes the characteristics of ERM in combination with the presence of a complete PVD or a partial PVD with fixation in the macular area. The average age of patients in the group with PVD and ERM was significantly higher than that of patients with ERM and pPVD, which indicates a prolonged process of PVD. In addition, significant differences were noted in the frequency of detection of radial retinal folds and a smoothed foveal profile according to the results of optical coherence tomography, which characterizes the severity of ERM manifestations. These findings prevailed in the group of patients with PVD. Thus, more pronounced manifestations of ERM—radial folding and smoothing of the foveal profile—are a specific and sensitive finding for patients with ERM and pPVD [23].
The role of the ILM
The ILM forms the innermost layer of the retina and is located on the border with the ST. The formation of the ILM involves the plasma membrane of the endings of the BM, possibly other retinal cells, such as astrocytes, as well as collagen fibers and ST proteoglycans [24].
In support of the first hypothesis of the occurrence of ERM, the origin of ILM defects remains unclear: whether they exist as a structural feature of the ILM or arise during PVD [15].
A. Gandorfer et al. [25] examined ILM samples removed from patients during surgical closure of an idiopathic macular hole. Using immunological markers for glial cells, hyalocytes and RPE, the authors discovered three pores in the ILM. The pores were a local complete defect of the ILM with irregular edges, in the projection of which the remains of glial cells were found. Considering the fact that glial cells are directly involved in the formation of the ILM, and the study does not provide the presence or absence of PVD as a factor in microtraumatization of the ILM, and also does not exclude iatrogenic damage to the ILM during surgical removal, the existence of natural pores in the ILM requires confirmation. Thus, given that pores in the ILM are an extremely rare [25] and controversial finding, it is probably not their presence, but other factors that play a large role in the development of epiretinal proliferation, which is common in the population.
Microcirculation disturbance
By definition, the development of iERM is not associated with preexisting disorders in the retinal vasculature. Nevertheless, at a certain stage of development of iERM, a disturbance of microcirculation in the retinal vascular system was recorded by a number of authors when studying the speed of blood flow in the capillary bed in the area of the macular zone [26, 27]. A study using fluorescein angiography in patients with iERM revealed a significantly lower blood flow velocity in the capillaries compared to that in the group of patients without iERM and in the group of patients after removal of iERM during vitrectomy [26, 27]. The authors explain the fact of microcirculation disruption by tangential traction of the retina, the existing iERM, which causes disruption of the correct course of blood vessels and their deformation, as well as morphological changes in the capillary endothelium [26]. Impaired microcirculation is not the primary link in the formation of ERM; however, hemodynamic disturbances caused by ERM may likely play a role in the progression of the disease, creating conditions for hypoxia, a powerful stimulator of proliferation of retinal glial cells.
Molecular mechanisms of ERM formation
The expression of specific growth factors, cytokines, and other biologically active molecules can stimulate the migration of glial cells and the formation of iERM. In the table
All biologically active substances discovered during the study of iERM are given.
Biologically active substances found in the iERM are produced by RPE cells [31], fibroblasts, endothelial cells [36], and glial cells [31].
Thus, the formation of the ERM is the result of complex intercellular interactions through molecular mechanisms. Along with RPE cells and fibroblasts, cells with potentially high migratory and proliferative capabilities, glial cells may be another important target for the prevention and treatment of ERM. Being a source of a large number of biologically active substances, retinal glial cells, according to P. Hogg et al. [37], may give a more effective migratory response to standard chemoattractants than fibroblasts and RPE cells.
In the future, with an understanding of the role of all cell types, it will be possible to create conditions for the management of patients with ERM using the necessary complex of cytokines and growth factors.
Treatment
The epiretinal membrane can only be treated surgically; treatment consists of its removal. The indication for surgery is a significant loss of central vision, which disrupts the patient’s usual lifestyle. Since removal of the epiretinal membrane is a complex manipulation, surgery is resorted to when the possible risk of intraoperative retinal damage in the patient is comparable to his existing visual impairment.
The first stage of the operation is the removal of the vitreous body (vitrectomy), which provides the doctor with access to the posterior pole of the eyeball. After this, the epiretinal membrane itself is removed. As with any abdominal surgery, there is a risk of various complications, both general (infection, bleeding) and more specific (increased intraocular pressure, lens opacification, retinal rupture and detachment).
With a favorable outcome of the operation, in most cases it is possible to improve the patient's visual acuity by 20 percent or more.
Causes
An epiretinal membrane can develop in humans against the background of certain ophthalmological diseases. Such diseases include:
- Diabetic retinopathy;
- Retinal tear;
- Vitreous detachment;
- Retinal vein thrombosis.
This process can be activated by hemorrhages and inflammatory processes in the eye. True, in most cases, the epiretinal membrane is an idiopathic phenomenon, the origin of which remains unclear. It is known that it contains retinal pigment epithelial cells, fragments of the vitreous body, collagen, as well as fibrocytes and macrophages and macrophages.
Advantages of the Moscow Eye Clinic
To treat and diagnose eye diseases, the clinic uses the most accurate equipment and effective methods recognized by leading clinics in the world. The level of training of the clinic’s specialists allows for interventions of any level of complexity. The Moscow State Conservatory employs recognized world-class specialists, professors and doctors of science with extensive practical experience.
The clinic deals with the treatment of epiretinal membrane and other operations on the posterior segment of the eye, candidate of medical sciences, vitreoretinal surgeon Oleg Evgenievich Ilyukhin. Over the years of professional activity, he has successfully performed thousands of complex vitreoretinal operations. Dr. Ilyukhin has repeatedly completed specializations in leading domestic and foreign eye clinics. Operations are also performed by a surgeon of the highest category, the chief physician of the clinic, Fomenko Natalya Ivanovna, who has successfully performed more than 12 thousand operations of various categories of complexity.
The doctors of the Moscow Eye Clinic provide all the necessary assistance seven days a week, from 9 to 21 hours, seven days a week. During treatment, preference is given to minimizing the number of visits and performing outpatient procedures whenever possible.
How does the disorder affect vision?
Since the membrane is located in close proximity to the macula, it is natural that the symptoms are directly related to the deterioration of visual function. The early stage of the pathology may be asymptomatic, but later the following signs begin to appear:
- a person sees surrounding objects less clearly;
- the image may seem “in the fog”;
- straight lines seem curved, the correct perception of objects is disrupted.
Even later, fibrosis begins, the structure of the epiretinal membrane changes, dense scar formations appear, and edema often develops. Such pathological changes can affect the photoreceptors of the retina, severely affecting vision. The risk of retinal rupture and detachment sharply increases, which can lead to vision loss. Severe forms of epiretinal membrane are characterized by the appearance of “floaters” or a dark “veil” before the eyes, diplopia, and rapid deterioration of visual acuity.
Epiretinal membrane treatment cost
The cost of treatment consists of diagnostic studies, the volume of therapeutic manipulations and procedures, which is selected individually for each specific patient. Prices for medical services at MGK are affordable and fixed in the contract. The cost of diagnosis and treatment of eye diseases can be found here.
We work for you seven days a week, seven days a week, from 9 a.m. to 9 p.m. You can ask our specialists any questions you have by calling the MGK hotline 8(800)777-38-81 (free for mobile phones and regions of the Russian Federation) and the number in Moscow 8(499)322-36-36 or online, using Skype consultation on the website.
Yakovleva Yulia Valerievna
Why is vitreal cavity tamponade needed?
In the process of filling the vitreal cavity with intraocular fluid, the removal of gelled hyaluronic acid causes a significant decrease in its viscosity. The pressure gradient decreases, which can cause detachment of the retinal pigment epithelium, spreading over its entire area.
After vitrectomy surgery, subclinical retinal tears quite often become unstable. Even small retinal tears, after several hours or days, can provoke total retinal detachment.
To ensure that the retina fits as tightly as possible and is securely fixed, retinal strengthening is used for 10-14 days using laser or cryoretinopexy. Pneumoretinopexy with SF6 (sulfur fluoride gas) or SF6 mixed with air often lasts up to 7-14 days, when this gas or an isobaric gas-air mixture when filling the vitreal cavity makes it possible not to resort to longer tamponade with C3F8 (perfluoropropane gas).
Symptoms
Regardless of the clinical picture, epiretinal membrane of the eyes manifests itself with the following general symptoms that you should pay attention to:
- The quality of visual functions gradually decreases. This disorder occurs in only one eye.
- Visible objects begin to distort.
- The surrounding objects begin to double. This manifestation persists even when one eye is closed.
Most often, such signs of the epiretinal membrane appear in the first stages of the disease and remain insignificant. This is associated with later diagnosis, since with minor changes patients prefer to do without the help of a specialist. With later development, patients experience the symptoms described above, as they provoke a deterioration in central vision.
Cost of the operation
In the case of progressive development, the pathology can provoke a regular decrease in the quality of vision. In such cases, there is a need for surgery. The operation includes vitrectomy of the vitreous with further removal of the membrane.
The cost of surgery when diagnosing epiretinal membrane of the eye can depend on many factors and the complexity of the disease. In different medical centers, the cost can vary from 25 thousand to 135 thousand rubles.
Tamponade of retinal tears with silicone oil
In a large number of cases of proliferative vitreoretinopathy, tamponade with silicone oil is required. In addition, surgery is often necessary in cases of giant retinal tears or large retinal defects. This is explained by the fact that silicone oil at the interface provides lower surface tension compared to a gas-air mixture or air. The tissues of the eye are not able to absorb it, so silicone oil can remain in the eye indefinitely.
Some doctors mistakenly believe that silicone oil has negative effects, in particular:
- Toxicity, so it must be removed after a few months.
- Deteriorates visual acuity.
- Silicone oil tamponade promotes the development of glaucoma.
In addition, there is a widespread misconception among specialists that the injection site is not important for tamponade with silicone oil. That it can be effective for holes in the macula. That compared to silicone oil with a viscosity of 5000 cSt, oil with a lower viscosity of 1000 cSt emulsifies more. Also, for some reason, it is believed that in patients with an intact capsule or posterior chamber IOL, it is advisable to perform a posterior peripheral iridectomy.
Silicone oil, in comparison with the gas-air mixture, is not able to increase its volume, thanks to this, patients with silicone vitreal tamponade are allowed to fly on airplanes. When retinopexy is performed around inferior tears, the tamponade is performed below the horizontal meridian. If the retina is torn on the temporal or nasal side, a person can sleep on his side.
For large tears in retinal tissue and macular holes, retinopexy is not advisable, as it can cause the development of proliferative retinopathy (PVR). If, after vitrectomy, retinal breaks are not clearly visualized, retinopexy is postponed until the subretinal fluid is completely removed, inflammation and edema are eliminated. This technique is somewhat reminiscent of the technology of “limiting the rhegmatogenous component to avoid retinopexy.” To perform this, silicone oil replaces air, not liquid (ZVSM, not ZZhSM).