What you need to know about orbital hemangioma
Facial cavernous hemangioma is a congenital benign formation located under the skin.
Capillary hemangioma: The most common tumor of the orbit in children (1% of all newborns).
Cavernous hemangioma: The most common tumor of the orbit in adults.
Vascular formation of the orbit:
Capillary hemangioma: A benign tumor consisting of a collection of capillaries with proliferation of endothelial cells (hemangioendothelioma).
Cavernous hemangioma: A non-neoplastic venous malformation with large cavities lined by endothelium.
Hemangioma in newborns is usually a simple hemangioma. The reasons are not exactly known, but there are several theories:
- Unfavorable environmental conditions
- Taking medications during pregnancy
- Maternal hormonal imbalance
Hemangioma in adults is formed due to abnormal proliferation of blood vessels. The reasons are also not fully understood. Degeneration of a tumor into a malignant one occurs extremely rarely, however, very often the surrounding tissues are compressed by the tumor, affecting an increasingly larger surface.
Symptoms of eye hemangioma
There are capillary and cavernous hemangioma of the eye, as well as mixed variants. Capillary hemangioma, or “strawberry” nevus, manifests itself in newborns in the form of a limited tumor-like growth of bright red color. Protrudes above the skin level, turns pale when pressed and increases in size when the child screams or cries. It is more common on the upper eyelid and may extend into the orbit. In this type of hemangioma, active growth is observed in the first year of a child’s life and stops by the age of two. Then a reverse regression of the tumor occurs with complete disappearance by 5-6 years. In rare cases, hemangioma of the eye can be combined with lesions of other organs and systems in the form of Kasabach-Merritt syndrome (characterized by thrombocytopenia, anemia and blood clotting disorders), or Maffucci syndrome (combined with chondromatosis of the arms, legs and curvature of the tubular bones).
Cavernous hemangioma, or “flaming” nevus, appears as a spot, clearly demarcated, soft in consistency, pink in color, and does not fade when pressed. They also occur in newborns. With age, it does not increase in size and does not go away on its own; the color of the spot changes to dark red or purple. The skin under the formation may roughen, hypertrophy, become knotty, loose, bleed or become inflamed. The lesion is segmental in nature, but bilateral nevi are also found. Combined lesions occur with extensive hemangiomas affecting the branches of the trigeminal nerve. They manifest themselves in the structure of Sturge-Weber syndrome (characterized by a disorder in the development of the vessels of the retina and the membranes of the brain).
Complications of eye hemangiomas include: mechanical ptosis (the large size of the tumor interferes with the normal functioning of the eyelid muscles), as a result of ptosis, obstructive amblyopia develops; strabismus (a neoplasm in the eye area prevents the full movement of the eyeball in any direction). A scar or hypopigmentation may remain at the site of the regressed tumor. Large ocular hemangiomas can be complicated by bleeding, infection or suppuration.
Which method of diagnosing orbital hemangioma to choose: MRI or CT
Selection method
- CT, MRI with gadolinium.
Is MSCT of orbital vessels with contrast informative for hemangioma?
Capillary hemangioma:
- Heterogeneous space-occupying formation without clear boundaries in the upper inner quadrant of the orbit
- During a dynamic study, a rapid, intense signal increase is observed after the administration of a contrast agent
- Microcalcifications may be detected.
Cavernous hemangioma:
- Well-defined, medium-density mass formation
- During a dynamic study, a gradual, moderate increase in the signal is observed.
What will MRI images of orbital vessels with contrast show for hemangioma?
Capillary hemangioma:
- Space-occupying lesion in the upper inner quadrant of the orbit
- Low signal intensity on T1-weighted image, high signal intensity on T2-weighted image
- Fuzzy boundaries
- Can infiltrate nearby structures
- Rapid, intense signal enhancement following gadolinium administration.
Cavernous hemangioma:
- Well-defined mass formation
- In 80% of cases it is located inside the cone formed by the extraocular muscles
- Hyperintense on T2-weighted image
- Isointense on T1-weighted image, slight signal enhancement observed after gadolinium administration
- The signal intensity on the T2-weighted image may be reduced due to the presence of (partial) thrombosis.
Distinctive signs of hemangioma
Cavernous hemangioma:
- A well-defined, enhancing mass located within a cone formed by the oculomotor muscles, with high signal intensity on a T2-weighted image.
In children
A tumor on the eye of a newborn has the ability to grow quickly, which is why such children are under constant medical supervision. Even the smallest formations should be observed. Pediatricians believe that parents should remain calm in this situation. The main thing is to contact a doctor in a timely manner to eliminate the problem and possible complications.
In almost 90% of small children, the formation on the eye is not treated, since it has the ability to disappear with age, but not always.
In a situation where rapid tumor growth is noted, it is necessary to begin emergency treatment, since in addition to a cosmetic defect, this can lead to loss of vision. Surgical intervention is performed as a last resort and only after the child reaches 4 months of age.
Treatment
Capillary hemangioma:
- Observation
- Glucocorticoids
- Laser therapy
- Surgical removal has conflicting results.
Cavernous hemangioma:
- Surgical removal
- In case of high risk of surgical treatment, a wait-and-see approach may be justified.
Hemangioma in children is often the cause of ridicule from other children, starting in kindergarten. Therefore, psychological support for the child is also necessary.
Causes of tumor
The thoracic and cervical spine are equally often affected by this type of neoplasm. There are several risk factors that lead to such growth:
- Injuries. After spinal fractures, protrusions, hernias, the cells themselves responsible for the restoration and growth of bone tissue may change. It is at the site of fusion that a benign type neoplasm arises.
- Bad heredity. Genetics is the second most common risk factor for most neoplasms. If one of your relatives has been diagnosed with neoplasms of a different nature, there is a great danger that the gene has been passed on to you - you need to especially carefully monitor your health.
- Congenital anomalies of the structure of the bone and cardiovascular systems, various pathologies of the vertebrae, as well as arteries and veins, are quite capable of leading to the occurrence of tumors of different sizes and locations.
- Hormonal instability. According to statistics, a change in the concentration of the female sex hormone estrogen often leads to the appearance of the disease.
- Lifestyle and ecology also leave an imprint of danger. For example, if you work under heavy loads and your spine is often under pressure, there is a potential for tumors to develop.
Possible complications and consequences
- Pain syndrome
- Suppuration of hemangioma
- Minor cosmetic defect after removal of hemangioma.
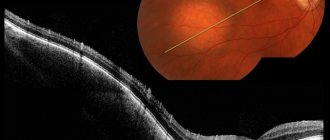
Hemangioma of the right orbit with hemorrhage into the tumor (arrow: fluid level). T1-weighted (a) and T2-weighted images (b) show a mass located within the cone formed by the extraocular muscles. The P-weighted image (c) after gadolinium administration shows a slight increase in tumor signal.
CT in the horizontal plane with contrast: contrast-accumulating retrobulbar hemangioma.
Prevention of eye hemangioma
Considering that eye hemangiomas are formed at the embryonic stage, preventive measures include careful monitoring and examination of women both during pregnancy and in preparation for conception. Several months before conception, it is necessary to undergo treatment of all chronic foci of infection (ENT organs, eyes, teeth). It is imperative to inform women about the dangers of smoking and alcohol during pregnancy, promptly release the pregnant woman from work in hazardous chemical industries or transfer her to easier work. In women over 35-40 years of age, it is necessary to carefully monitor blood pressure to prevent the occurrence of eclampsia. If an eye hemangioma is detected in a newborn, it is necessary to contact an ophthalmologist as quickly as possible for examination and clarification of the diagnosis, and also strictly follow the doctor’s recommendations.
LiveJournal
Results and discussion
In both groups of patients with WHC (76 people), signs of the disease were diagnosed in the 4th–5th decades of life (31–83 years). Similar information is presented in the literature [3]. There were slightly more women in the analyzed group (F:M - 1.24:1). Along with this, there is information both about the absence of gender differences in patients with AHC [18] and about the predominance of men among them [6]. Considering the largest number of observations (200 patients) presented in the work of CL Shields et al. [18], we must agree that gender differences do not play a role in the refined diagnosis of WHC.
The timing of diagnosis from the moment of appearance of the first signs of WHC (impaired visual functions) is shown in Table 1. The tumor was detected by chance (during the selection of glasses for work, during medical examination) in half of the patients (51.2%). Just over 1/3 of patients noticed symptoms of visual disturbance within 2–3 weeks. Before contacting a doctor, half of the patients had an anamnesis of no more than 1 year.
In total, of the 76 patients with WHC, 75 had a tumor that was monolateral and monofocal, predominantly affecting the center of the fundus (Table 2). In 1 patient, diffuse HC with Sturge-Weber syndrome was diagnosed in early childhood, and at the age of 8 years, a circumscribed HC nodule was detected.
The dimensions of the WGC in both groups at the time of clarification of the diagnosis reached 4.2 mm in thickness and 14 mm in maximum diameter. Median follow-up of patients was 5 years (1–14 years). Both in early publications and in publications of recent years, at the time of diagnosis, the size of the WGC turned out to be slightly higher; tumor prominence ranged from 1 to 8 mm, diameter - from 3 to 19 mm [18].
WHCs have their own distinctive ophthalmoscopic
picture
. The tumor is colored red (Fig. 1A), sometimes with a yellowish tint (Fig. 1B). Its shape is round or oval, the surface is smooth. As the size of the WGC increases, areas of whitish-gray color may appear on its surface (Fig. 2).
The boundaries of the WRC at small sizes are quite clearly contoured. At this stage, the subretinal exudate is weakly expressed, the symptom of “openwork” of the tumor is ophthalmoscoped (Fig. 3), which is associated with the formation of cysts in the retina. In 13 of our cases (17.1%), the tumor had a dark red color with a slate tint, which can be explained by ongoing changes in the overlying retina (reactive metaplasia of the pigment epithelium) (Fig. 4). The pigmented zone around the WGC, which is ophthalmoscopically visible in some cases, is most likely due to compression of choroidal melanocytes along the tumor border (Fig. 5) [2] .
In 6 patients with a history of visual disorders of less than 1 month. (2 eyes), 12 months. (3 eyes) and 12 years (1 eye) OHC was accompanied by extensive retinal detachment.
According to M. Furuta et al., stable subretinal fluid above the RGC leads to pathological changes in the neurosensory retina when the tumor persists for 3 months or more. and more [19].
In 10 cases, a zone of chorioretinal atrophy with pigmented areas on the surface was detected around the tumor. According to OCT data, in these areas there was atrophy of the choriocapillaris with hyperreflective areas above Bruch's membrane and the outer layers of the retina (Fig. 4).
Visual disturbances in WHC are characterized by a hypermetropic shift without changes in the overlying retina [20], or gradual blurring of vision, or sudden loss of vision, which is associated with secondary changes in the retina [2].
In 9 observations, the hypermetropic shift caused by prominent WHC was from 0.5 to 1.5 diopters. (on average 0.83±0.13 diopters) compared to the fellow eye. Maximum corrected visual acuity (BCVA) varied from 0.3 to 1.0 (average 0.66±0.07), the average thickness of the WHC was 1.79±0.24 mm. In all patients, hypermetropization was combined with developed changes in the outer layers of the retina (OCT data).
Among our patients with central localization of OHC (29 people), a decrease in vision to 0.45 ± 0.07 occurred in 19 cases, of which in 11 cases subfoveal detachment of the neurosensory retina was detected. In 8 eyes, damage to the photoreceptor layer (edema or atrophy) in combination with edema of the nuclear layers or cystic changes in the retina in the macular zone occurred in the foveal zone.
The biometric parameters of the tumor in the group with preserved vision (1.0) and the group with reduced vision are comparable: average prominence - 2.06±0.2 and 2.21±0.18 mm (p=0.14), average diameter - 7.15±0.76 and 7.09±0.51 mm (p=0.53) with preserved and reduced visual acuity, respectively. A direct correlation was revealed between BCVA and the distance from the edge of the WGC to the fovea (r=0.43; p=0.03) - in cases with decreased visual acuity, the WHC were located significantly closer to the fovea (p=0.03) on average by 1308, 71±417.69 µm.
Thus, with GC of central localization in groups with preserved and reduced visual acuity, the biometric parameters of tumors do not differ in prominence. It should be noted that in cases with reduced vision, subfoveal retinal detachment was detected, the height of which correlated with the biometric parameters of the WRC (r=0.39; p=0.02 and r=0.45; p=0.01 for thickness and diameter, respectively ).
Incidentally detected WHC is not such a rare occurrence. The frequency of such cases in the observed group was almost half (50.1%). In 11 of them, with a follow-up period of 1–12 years (median 5 years), visual acuity remained within 1.0 when the tumor was localized in the macular zone; in 2 of these cases, the tumor was located directly under the fovea. It is believed that a violation of the architectonics of the retina with structural changes (cystic dystrophy) occurs when the thickness of the retinal retina is more than 1.8 mm, and retinal edema occurs when its thickness is greater [5]. At the same time, there are publications describing WHC, presented on OCT in the form of a dome-shaped elevation of the choroid with focal hyperplasia of the pigment epithelium and serous retinal detachment above the tumor with preserved retinal architecture and a normal photoreceptor layer [21]. In our observations, edema at the level of the nuclear layers of the retina occurred when the thickness of the retina was 1.34 mm or more, and when the thickness of the retina was more than 1.65 mm, cysts appeared. Dynamic observation showed that edema at the level of the nuclear layers precedes the appearance of cysts (Fig. 6).
A decrease in visual acuity occurs with the appearance of intraretinal microcystic edema or neurosensory retinal detachment [6, 22].
In our observations, with central localization of the tumor, BCVA was reduced to an average of 0.48±0.08 in 15 cases. However, only 9 of them showed subfoveal detachment of the neuroepithelium. In other cases, the edge of the tumor was located in the foveal zone, and in the overlying retina there was swelling of the photoreceptor layer in combination with edema or cysts at the level of the nuclear layers. In 2 eyes, atrophy of the photoreceptor layer was detected, which was the cause of decreased vision.
It should be noted that in 1 case, BCVA persisted for 2.5 years. During the observation process, the appearance of edema of the nuclear layers in the foveal zone, as well as a gradual increase in cysts in the parafoveal zone, was noted. Subsequently, the patient developed subfoveal detachment of the neuroepithelium, and an increase in cystic edema was also noted, spreading to the foveal area (Fig. 6).
This case allows us to explain the complaints of fluctuations in visual disturbances inherent in a number of patients with WHC. Stable visual impairment occurs with gross destruction of the photoreceptor layer and pronounced cystic changes in the proper layers of the retina.
Do changes in the overlying retina depend on the thickness of the WGC? In the observed group of patients with incidentally detected WCC, 11 patients with an average tumor thickness of 2.13 mm (0.8–3.6 mm) had a visual acuity of 1.0 maintained over an average follow-up period of 4.46 years (1–12 years). At the same time, in 10 patients who complained of decreased vision, WHC with an average thickness of 2.34 mm (0.83–4.2 mm) was diagnosed. Signs of decreased vision appeared on average 3.6 years (1–9 years) before visiting a doctor. In general, in this group (17 eyes), the OHC was localized paramacularly, involving part of the macula; in 2 eyes it was located directly under the fovea, in 2 eyes it was juxtapapillary above the optic nerve head. There was no statistically significant difference in GC prominence in the group with preserved and reduced vision (p=0.72).
It is known that WHC begins its development in the outer layers of the choroid [1]. The extravasation of the liquid fraction of blood and retinal edema during acute retinal retinal edema are based on the characteristics of its blood flow. Studies of OCT angiography of eyes with WGC have proven the presence of a peculiar vascular pattern with unevenly dilated large vessels in the area of interest [23] and preservation of the choriocapillaris zone [24]. Previously, the vessels of the WHC itself were studied; their diameter turned out to be 2.5–5 times larger than the diameter of a normal choroidal vessel on the contralateral side, and the blood flow in the hemangioma itself was slower than in other choroidal tumors [25]. By analogy with cavernous angiomas of the brain, which have low blood flow, WHC can be regarded as tumors with passive blood flow [26, 27]. It is these features of blood flow in the RGC that underlie the extravasation of the liquid fraction of blood and retinal edema. This process is usually slow
Total retinal detachment and complete blindness of the eye are the outcome of a gradually increasing WCC. The issue of treatment of WCC, as shown by an analysis of the literature, is ambiguous: indicators such as the initial size of the tumor, its location, the condition of the proper retina and the treatment technique owned by the supervising physician play an important role . But already in 1997, the advantage of BT was shown as an effective method of resorption of subretinal fluid [28]. Using OA with gamma sources, an additional decrease in the thickness of the WGC was observed by an average of 50% after 1 course of irradiation [14].
Having experience in the treatment of choroidal tumors with BT, including WHC, we retrospectively assessed the effectiveness of this treatment method in 35 patients with WHC after a single course of BT with a follow-up period of 18–24 months. The initial maximum prominence of the WGC was 4.5 (median - 3.47 mm), maximum diameter - 10.6 mm. The juxtapapillary localization of OHC prevailed (40%), the paramacular and nasal half of the fundus were almost equally distributed (31.43 and 28.57%). Initial visual acuity was reduced when the tumor was centrally localized (10 eyes) and juxtapapillary - on the upper outer edge of the optic nerve head (7 eyes). In the remaining 18 eyes, vision remained high (0.8–1.0). Based on the presented data, the result of BT was assessed by the degree of resorption of subretinal fluid and the state of the WGC itself. Complete resorption of subretinal fluid and tumor (replaced by a flat chorioretinal scar) occurred in 18 eyes within 12–16 months. 1 year after irradiation, in 16 eyes, the subretinal exudate was completely resorbed, and the prominence of the hemangioma decreased by half its original thickness. The positive effect lasted for 18–24 months. follow-up (median: 22 months). In 1 case, with a 4.25 mm prominence of the retinal retina with widespread retinal detachment and a sharp decrease in vision for 8 years, the result of BT was negative. A reduction in tumor thickness as a result of its partial regression after BT was also observed by other authors [14]. Complete or partial resorption of WGC is possible against the background of deteriorated blood flow in newly formed tumor vessels, and it is possible that as a result of radiation damage to the intravascular endothelium with subsequent complete blocking of blood vessels. This was confirmed by OCT angiographic studies, which showed a statistically significant decrease in the area of the blood circulation vessels and the blood flow in them, recorded 1 year after BT [29]. Despite the fairly rapid resorption of subretinal fluid and retinal reattachment, the preservation of the RCC, including residual tumor, is often the cause of recurrent retinal detachment [28]. Both after laser coagulation and TTT, and after BT, late complications are possible [30]. These include radiation retinopathy (24%), radiation papillopathy (5%) and subretinal fibrosis (10%) [31]. These are, as a rule, late complications that appear almost 2 years after irradiation of the eye [14]. Radiation maculopathy developed in 8 of our patients (22.86%) with WHC near the macular area. Such a high incidence of maculopathy after BT of any tumor in the central zone of the fundus is understandable: the diameter of the macular zone, which must be preserved during paramacular WHC, is small - only 500 µm [32]. That is why this complication should be regarded as planned.
The question arises: should WHC be treated and when? G. D. Lewis et al. It is believed that a long follow-up period for WHC and decreased visual acuity before treatment are risk factors for poor visual outcome [8]. It is difficult to argue against this, given the destructive changes in the retina above the WGC detected on OCT. At the same time, as our observations show, BCVA in macular localization of GC can remain preserved for a long time: in 11 patients with a fairly long follow-up period (median 5 years), BCVA remained equal to 1.0. Along with this, in half of the patients who complained of decreased vision (11 people), the diagnosis of acute hyperthyroidism was made at the first visit, and the duration of decreased vision (up to 0.6–0.7) was short (less than 1 year). An explanation for this can be found in the literature: OCC localized in the fovea and macula can remain asymptomatic for a long time, even with changes in the retina, due to the extremely slow progression of the tumor [24].
Diagnostics
For an initial diagnosis of hemangioma, a visual examination is sufficient for an experienced specialist. The clinical picture of the disease is usually pronounced. To diagnose a neoplasm on the eyelid, it is possible to use biomicroscopy.
If necessary, various research methods are used to clarify the diagnosis and differentiate the pathology from other vascular tumors. If it is necessary to study the structure and depth of tissue damage by hemangioma, a biopsy, ultrasound examination (ultrasound) of the eyeball or MRI is used.
The angiography procedure helps to clarify the boundaries of formation and the extent of distribution of pathological vessels. X-rays, computed tomography and echography are also effective in diagnosing hemangioma.
A vascular tumor on the eyelid should be differentiated from thrombosis of the cavernous sinus, lymphangioma, cyst, cerebral hernia, and perinatal hematoma.