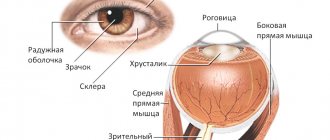
The central part of the retina (macula, or macula) is represented by nerve fibers and a thin layer of cells that are sensitive to light and color (the concentration zone of cone photoreceptors, which form central vision). Thanks to the presence of cones, a person sees during the day and distinguishes colors. Because the cells of the macula contain yellow pigment, it is also called the “yellow spot.” Patients suffering from diabetes mellitus and other general diseases develop macular edema. It leads to a decrease in visual acuity or loss of vision, including complete blindness.
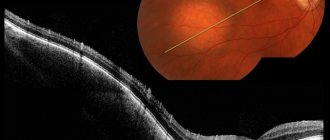
Fig. 1 Retina of a healthy eye and macular edema against the background of diabetic retinopathy
Causes and factors contributing to the development of macular edema
Macular edema is not an independent disease. It may be a manifestation of the following pathology:
- Diabetic retinopathy (damage to retinal vessels due to diabetes mellitus);
- Damage to the eyeball or consequences of surgery (for example, cataract removal);
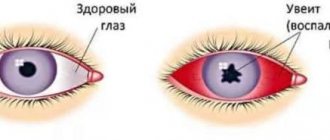
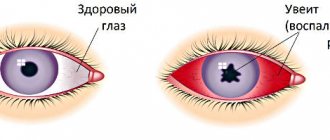
- Uveitis (inflammation of the choroid of the eye);
- Blockage (occlusion) of the central retinal vein (CRV) and its branches.
The main causes of the development of macular edema are diabetic retinopathy and retinal vein occlusion due to arterial hypertension and atherosclerosis. Less commonly, surgery or trauma.
If the retinal veins are blocked without immediate treatment, irreversible vision loss can develop!
The following factors contribute to the formation of macular edema:
- Hyperglycemia – increased blood glucose levels;
- Hyperlipidemia is an abnormal increase in lipids and cholesterol in the blood;
- Excessive body weight;
- Insufficient physical activity (hypodynamia);
- Age over forty or fifty years;
- Increased intraocular pressure, blood viscosity;
- Inflammatory diseases of other organs and systems.
An important factor in the development of macular edema is a congenital and acquired predisposition to the formation of blood clots. When a blood clot forms, the blood flow through the vessel is disrupted. Formed elements of blood penetrate into the retina, which leads to its thickening and swelling. As a result of thrombosis, a zone of ischemia—“oxygen starvation”—develops around the affected area.
In order to improve blood flow, new vessels with pathologically thinned walls begin to grow into the retina from the underlying choroid (choroid). Through them, the liquid part of the blood (plasma) penetrates into the retina. When blood pressure rises, the abnormal walls of the vessels rupture and blood with formed elements pours into the retina. The swelling area expands. The macula ceases to perform its main function - providing high-quality central vision. If the patient does not receive timely medical care, the retinal receptors die, and he may lose his vision forever.
The two most common types of edema are diabetic and cystic. The first develops against the background of diabetes, the second - after eye surgery (it is also called (Irvine-Gass syndrome). Below we will look at diabetic macular edema (DME), you can read about cystic HERE.
Symptoms of DME
Diabetic macular edema is manifested by the following symptoms:
- Blurred image in the central field of view;
- Image distortion (straight lines look curved, wavy);
- Increased sensitivity to light.
Patients focus on the appearance of a pink tint to the image. They note a decrease in visual acuity at certain times of the day, often in the morning. The perception of color can change throughout the day.
Fig. 2 Vision of a sick person with macular edema
Diagnostics
Severe macular edema is diagnosed by ophthalmologists during an examination of the fundus of the eye (procedure - ophthalmoscopy). If the disease is detected at this stage, a favorable outcome does not always occur. Treatment takes a long time and requires a lot of effort.
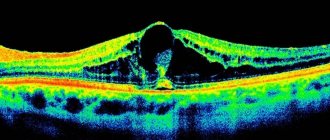
Predisposition to the development of the disease or the early stage of the pathological process is identified using optical coherence tomography (OCT). We recommend that people who have factors that contribute to the formation of retinal vein blood clots undergo this procedure once a year. Detection of macular edema at an early stage allows timely emergency measures to be taken to remove it and preserve vision.
Fig. 3 Picture of macular edema on OCT - optical coherence tomography
In our clinic, accurate diagnosis of retinal diseases is carried out using the new generation optical coherence tomograph DRYOCT Triton, which has an angiography function. It allows you to obtain a two-dimensional and three-dimensional image of the structure of the retina and the structures of the optic nerve head, examine the vessels of the retina (if necessary, determine the location of the leakage without resorting to the injection of a contrast agent).
When using this research method, macular pathology is detected in the early stages. In this case, the forecast is optimistic.
Orbital tumors
Orbital tumors account for about 1/4 of all tumors of the organ of vision. Among them there are both benign (80%) and malignant (20%) processes.
Metastatic tumors can develop in the orbit!
Symptoms of an orbital tumor: pain in the orbital area, decreased visual acuity and double vision, displacement and limitation of eye mobility, swelling of the mucous membrane of the eye and eyelids, palpable formation under the skin, dysfunction of the eyelids (drooping, deformation)
It should be remembered that orbital tumors can be asymptomatic
Methods for diagnosing orbital pathology:
1. Ophthalmological examination
2. Viso-, peri-, campi-, ophthalmometry
3. Exophthalmometry – non-invasive determination of the degree of displacement of the eyeball relative to the bony walls of the orbit
4. Ultrasound (including with Doppler examination)
5. X-ray computed tomography and magnetic resonance imaging are leading non-invasive diagnostic methods
6. Positron emission tomography
7. Biopsy is the leading method of invasive diagnosis of orbital tumors. Morphological methods for diagnosing orbital tumors: excisional biopsy, incisional biopsy, fine-needle aspiration biopsy.
The most rational type of intervention is determined by the doctor at the stage of preparation for surgery.
Only in our institution a modern diagnostic method is performed - trephine biopsy.
Surgical methods for the treatment of orbital tumors, available in the arsenal of ophthalmic-oncologists, are carried out only in cases where there is no destruction of the bone walls and the tumor has spread to adjacent areas. Otherwise, the patient should be sent to a specialized oncology hospital.
Types of treatment for orbital tumors:
Depends on clinical symptoms, characteristics of the pathological process (the expected nature of the neoplasm, type of growth, size, location) and is determined by the doctor
1. Surgical treatment is the most commonly used type of treatment. Complete removal of the formation is carried out in cases of limited single lesions in the orbit
Removal ad maximum followed by radiation or chemotherapy is carried out in cases of diffuse tumor growth, multiple foci, or suspicion of a specific type of tumor (for example, lymphoma, metastases) that will require further chemo-radiation treatment.
Orbital exenteration (complete removal of the soft tissue contents of the orbit to the bone walls) is a mutilating type of surgery, performed in cases of confirmed malignant process in the orbit, occupying a large part of the orbit, when no other treatment method is indicated. External prosthetics can be performed within about 9-12 months after surgery.
2. Radiation therapy - some types of tumors and inflammatory diseases
3. Chemotherapy - the need for this treatment is determined by an oncologist during a consultation in a specialized institution. It is usually necessary in the treatment of metastatic tumors, lymphomas, etc.
4. Combined treatment.
5. Conservative treatment – treatment with medications and physiotherapy. Indicated for pseudotumor (inflammatory) processes, vascular pathology of the orbit.
Condition after a course of conservative treatment (decongestant, vaso-strengthening, anti-inflammatory, desensitizing drugs).
Observational tactics are possible in cases of a small tumor, presumably benign, with very slow growth (years), which does not affect the visual acuity and cosmetic appearance of the patient.
Treatment
Treatment of diabetic macular edema is often carried out by ophthalmologists together with endocrinologists. Patients are prescribed a special diet and medications that normalize blood glucose levels.
With conservative (drug) therapy, patients are prescribed anti-inflammatory drugs in the form of injections, tablets, drops.
Laser coagulation
The most effective method of getting rid of most retinal diseases (dystrophy, rupture, detachment) is laser coagulation, this also applies to DME. However, this method of treatment for macular edema requires careful and balanced use, since laser beams can damage the macula. Therefore, coagulants (if there is such a need) are applied along the edge of the central zone of the retina to prevent further spread of edema.
Intravitreal drug administration (IVD)
Depending on the cause of macular edema, intravitreal (intraocular) injections of various drugs may be prescribed. This delivery method is the most effective in the treatment of retinal diseases.
The first type of drugs is anti-VEGF (vascular endothelial growth factor blockers). These include ranibizumab (Lucentis), aflibercept (Eylea), and bevacizumab (Avastin). The latter is not approved for use in ophthalmology in Russia, but some clinics use “Off Label”, i.e. not according to the indications specified in the instructions. Anti-VEGF reduces swelling and prevents its recurrence.
Drugs that contain synthetic analogues of glucocorticosteroids (hormones synthesized by the adrenal cortex) are highly effective in treating DME caused by retinal vein thrombosis. To get maximum results, they are injected directly into the vitreous body. Despite the high effectiveness of corticosteroid injections compared to other pharmacological forms (drops, tablets), the duration of the therapeutic effect in many cases is short due to the fact that the main active substance is absorbed immediately.
This is not observed when the innovative drug Ozurdex is implanted into the vitreous body, which is made in the form of a depot form (implant) and releases dexamethasone in doses over several months.
Our clinic has all the capabilities to treat diabetic macular edema even in the most complex cases. We offer fast and highly accurate diagnostics and the opportunity to begin treatment (including intravitreal injections) with retinal specialists on the same day of your visit!
Angioid retinal streaks
- Classification
- Diagnostics
- Fluorescein angiography of the retina
- Treatment
Angioid retinal stripes (dystrophy or Grönblad-Strandberg syndrome, generalized elastorexis, dystrophic elastosis, elastic pseudoxanthoma) are a very peculiar and extremely rare form of fundus pathology, resembling retinal vessels in appearance. These are actually linear cracks that form in Bruch's membrane.
Generalized elastorexis is a hereditary systemic lesion of the elastic tissue of the skin, retina and blood vessel walls. In half of the patients, angioid stripes of the retina are found. 70% of patients with retinal angioid streaks develop loss of central vision.
In 60% of cases, angioid streaks are associated with systemic elastorhexis and in this case are called Grönblad-Strandberg dystrophy (or Grönblad-Strandberg syndrome).
In other cases, angioid stripes are an independent disease. A combination of angioid bands with sickle cell anemia and Paget's disease is possible, but information about this is extremely scarce.
This nosological form was first described by Doyne (in 1889), and somewhat later Grenblad and Strandberg established a connection between this pathology and a systemic disease of elastic tissue - elastorexis. It is based on the insufficiency of the formation of coenzymes that inhibit elastase.
The disease is rare and has not yet been sufficiently studied. Both familial cases of the disease and sporadic forms have been described.
The disease is generalized in nature with primary damage to the vessels of the heart and brain, as well as the gastrointestinal tract, often with recurrent, sometimes profuse bleeding. A common manifestation of the disease is obliterating endarteritis. Endocrine disorders (diabetes mellitus, lesions of the thyroid gland, pituitary gland), pregnancy, infectious diseases, the presence of foci of chronic infection, toxoplasmosis, and trauma can play a provoking role.
The frequency of the disease in the population is 1:160,000. 2 recessive and 2 dominant types of the disease are described, differing in concomitant anomalies.
- Elastic pseudoxanthoma dominant, type 1 (177850, PXE gene, VD). Clinically: thickening of the skin, orange peel skin, striae, calcification of large and coronary arteries, bleeding from the gastrointestinal tract, calcification of the dura mater.
- Elastic pseudoxanthoma dominant, type 2 (177860, R). Clinically: thickened nodular or reticulated skin of a yellowish color, areas of decreased skin elasticity, myopia, blue sclera, high arched palate, occurs 4 times more often than dominant type 1. Laboratory: elastic fibers contain small areas of calcification.
- Elastic pseudoxanthoma recessive, type 1 (264800,16p13.1, PXE1 gene, p). Clinically: symptoms of dominant type 1 in combination with cardiomyopathy and mitral stenosis. Laboratory examination: fragmentation of elastic dermal tissue.
- Elastic recessive pseudoxanthoma, type 2 (264810, p) is a very rare form. Clinically: the skin is compacted, thickened, but without stretch marks, there are no vascular changes.
Doyne associated retinal angioid stripes (AR) with ruptures of the pigment epithelium, and Coffer (1917) with ruptures of Bruch’s membrane. Electron microscopic studies showed damage to the elastic layer of Bruch's membrane, swelling and splitting of elastin, followed by destruction and disintegration. Elastic fibers turn into lumps and grains with the deposition of calcium salts in them, which ultimately leads to tears and ruptures of Bruch's membrane.
At the same time, dystrophic changes in the retinal pigment epithelium and choriocapillaris are detected. Detachment of the pigment epithelium with exudate and blood is possible.
The localization of the disease is bilateral with varying degrees of severity of manifestations.
Ophthalmoscopically, angioid stripes look like “vascular tracks” running concentrically around the optic disc, forming a ring or semi-ring, from which they diverge radially to varying lengths, forming a kind of vascular tree around the optic disc.
Retinal vessels, passing over the angioid stripes, do not change their course. The color of AP is from pale pink to brownish-brown, the edges are uneven. The diameter ranges from 1 to 2 times the diameter of a large retinal vein, and the outline is slightly jagged.
The origin of hemorrhages is usually choroidal, less often – retinal; in isolated cases, a disc-shaped pseudotumor lesion can form (like Kunt-Junius dystrophy).
In young people, APs have a more delicate pink color and are less contrasting in relation to the main background of the fundus. Their length, as a rule, does not reach the macular zone.
Visual functions can be high, up to 1.0. With age, APs become more contrasting and pigmented, acquiring a dark brownish-brown color. There is a gradual increase in their width and length, and the appearance of new branches. Changes in the retina and choroid can be detected not only in the AP area, but also diffusely throughout the fundus.
Classification
Shulpina N.B. et al. distinguishes 3 stages during the disease:
- the appearance of striae around the optic nerve head;
- spread of striae to the macular regions and the occurrence of hemorrhages;
- a scarring process with choroidal atrophy and deposition of pigment clumps.
Mizgireva A.P. identified 2 types of changes in the central zone:
- focal-diffuse chorioretinal dystrophy, occurring in the dry type (uncomplicated form); Slowly progressive atrophy of the choriocapillaris layer, as well as secondary changes in the pigment epithelium and outer layers of the retina. There were no hemorrhages or edema in the macular area in patients of this group; visual functions remained quite high.
- chorioretinal dystrophy, occurring according to the “wet type” (complicated form). Formation of subretinal neovascular membrane (SNM). The clinical picture in these patients was determined by the destruction of Bruch's membrane and the germination of newly formed vessels, followed by recurrent hemorrhages and edema with detachment of the pigment epithelium and neuroepithelium. The newly formed vessels originated from the choriocapillaris and had a capillary type of structure. Growing through the vitreous plate, they appeared first in the outer layers of the retina, then in the inner ones. The edema was transudative in nature, originating from the choriocapillaris and newly formed vessels. The hemorrhages had the same genesis. The retinal vessels, as a rule, remained intact, i.e. SNM developed. FA in these patients was typical for SUI, depending on the degree of its manifestation. Only in a third of cases, the SUI is closely associated with the AP, the distal end of which passes into the hyperfluorescent zone of the SNM. However, sometimes even angiography does not reveal signs of the presence of an AP near the area of SUI formation. Visual functions are usually low. A relapsing nature of the course is observed. Each exacerbation is accompanied by the development of glial elements with the subsequent formation of glial foci, which can occupy the entire macular area and proliferate, having the appearance of pseudotumor formations. Subsequently, a scar forms in the center, involving the foveal area, which significantly reduces central visual acuity.
It is noted that type 1 macular changes can turn into type 2 with age, and in some cases, with a high degree of probability, the provoking factor can be considered an eye contusion, after which the process proceeded as type 2.
Diagnostics
The diagnosis is based on the clinical picture; in doubtful cases, a histological examination of the affected skin is carried out (foci of degeneration and decay of elastic fibers of the dermis and their infiltration with calcium salts).
Fluorescein angiography of the retina
In the choroidal or early arterial phases, hyperfluorescent tracks with uneven but clear contours are detected; the peak of hyperfluorescence coincides with the arteriovenous phase and lasts for a long time, gradually weakening towards the end of the study.
The timing of the arterial and venous phases of the retinal circulation, as well as the timing of arterial and venous perfusion, may be normal, but, as a rule, there is a prolongation of the early venous phase and venous perfusion.
In uncomplicated forms, there is no extravasation of fluorescein from the retinal vessels. In general, the angiographic picture of the fundus in the presence of angioid streaks is very clear and specific for this pathology.
Treatment
Treatment for AP has not been developed and, according to some authors (Shulpina N.B. et al.), is ineffective. A positive clinical effect was noted from the use of ACTH and prednisolone.
Antioxidants (emoxypine, tocopherol), parabulbar administration of ethamsylate, electrophoresis of cysteine and 3% potassium iodide, as well as argon laser coagulation of cracks in Bruch's membrane are recommended.
When hemorrhages occur, hemostatic, decongestant and absorbable agents are used.
It is necessary to prevent atherosclerotic changes that worsen the course of the main process.
Patients should be under medical supervision of a dermatologist, ophthalmologist, or therapist.